Reduced Birth Weight and Exposure to Per- and Polyfluoroalkyl Substances: A Review of Possible Underlying Mechanisms Using the AOP-HelpFinder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Development of the Search Term Lists
2.2. Running the AOP-helpFinder Tool
2.3. Manual Curation
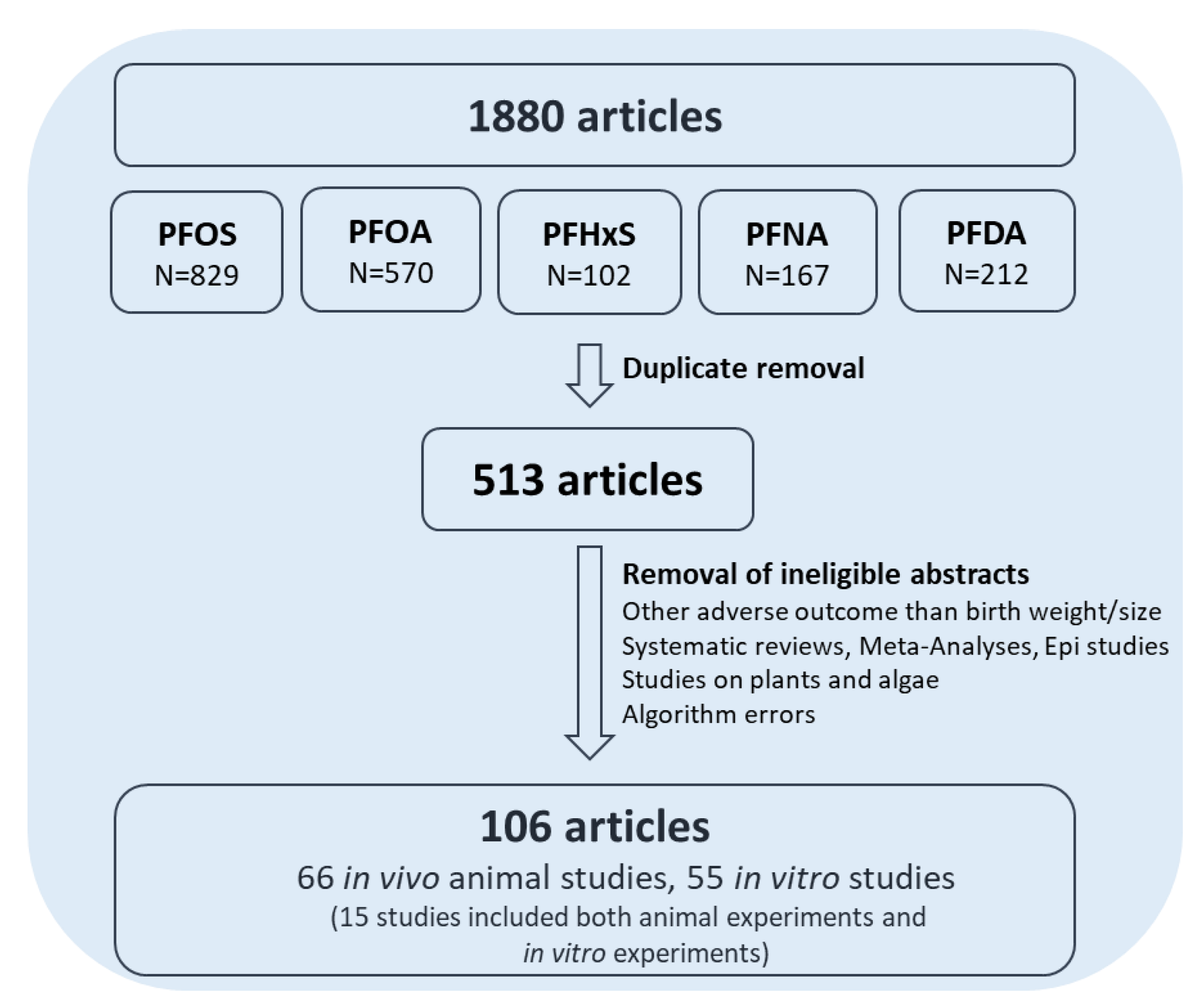
2.4. Flowchart
3. Results
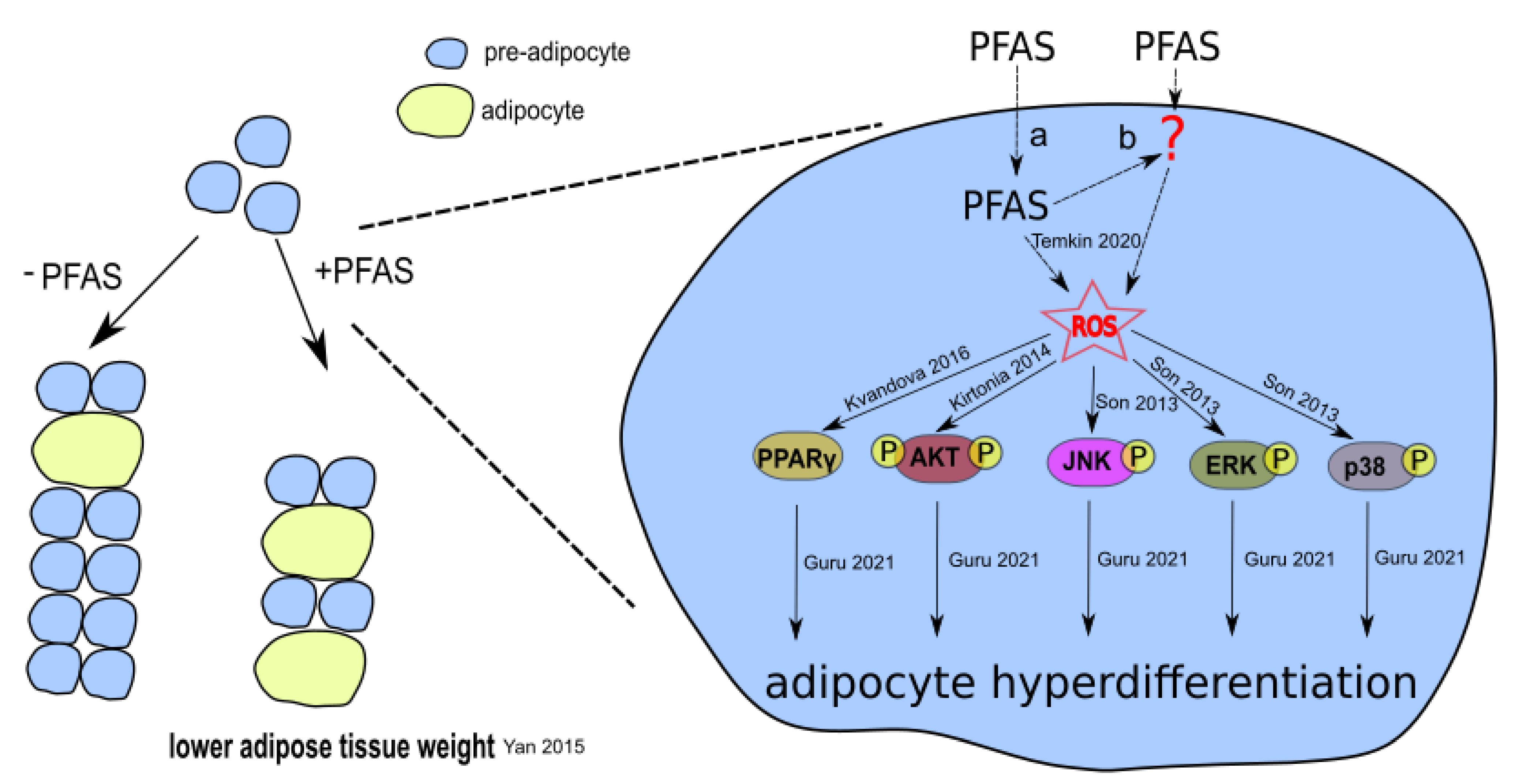
3.1. PFAS-Associated Cytotoxicity and Oxidative Stress
3.2. PFAS-Associated Activation of PPAR, AKT, and MAPK Signaling Pathways
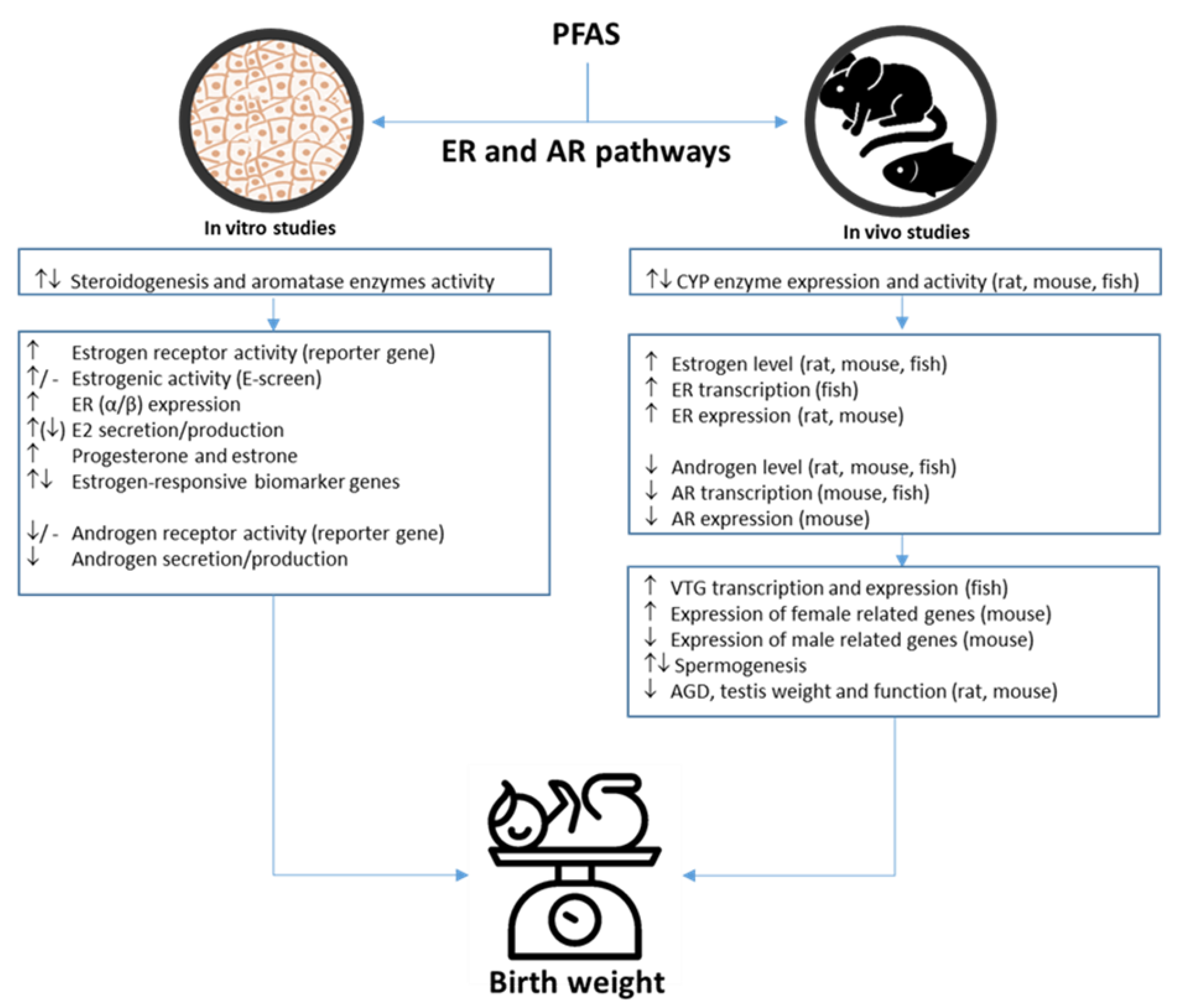
3.3. PFAS-Associated Endocrine Effects
PFAS-Associated Estrogenic and Androgenic Effects
4. Discussion
4.1. Experimental Studies on Oxidative Stress and Cytotoxicity
4.2. PFAS-Associated Endocrine Effects
5. Strengths and Limitations of the Study
6. Conclusions and Future Experimental Model Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savitz, D.A. Guest editorial: Biomarkers of perfluorinated chemicals and birth weight. Environ. Health Perspect. 2007, 115, A528–A529. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.J.; Pool, R.; van de Weijer, M.; Chen, X.; Krapohl, E.; Gordon, S.D.; Nygaard, M.; Debrabant, B.; Palviainen, T.; van der Zee, M.D.; et al. Genetic meta-analysis of twin birth weight shows high genetic correlation with singleton birth weight. Hum. Mol. Genet. 2021, 30, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Brundtland, G.H. From the World Health Organization. Reducing risks to health, promoting healthy life. JAMA 2002, 288, 1974. [Google Scholar] [CrossRef] [PubMed]
- Finken, M.J.J.; van der Steen, M.; Smeets, C.C.J.; Walenkamp, M.J.E.; de Bruin, C.; Hokken-Koelega, A.C.S.; Wit, J.M. Children Born Small for Gestational Age: Differential Diagnosis, Molecular Genetic Evaluation, and Implications. Endocr. Rev. 2018, 39, 851–894. [Google Scholar] [CrossRef] [Green Version]
- Ludvigsson, J.F.; Lu, D.; Hammarström, L.; Cnattingius, S.; Fang, F. Small for gestational age and risk of childhood mortality: A Swedish population study. PLoS Med. 2018, 15, e1002717. [Google Scholar] [CrossRef] [Green Version]
- Govarts, E.; Iszatt, N.; Trnovec, T.; de Cock, M.; Eggesbø, M.; Murinova, L.P.; van de Bor, M.; Guxens, M.; Chevrier, C.; Koppen, G.; et al. Prenatal exposure to endocrine disrupting chemicals and risk of being born small for gestational age: Pooled analysis of seven European birth cohorts. Environ. Int. 2018, 115, 267–278. [Google Scholar] [CrossRef] [Green Version]
- Jensen, E.A.; Foglia, E.E.; Dysart, K.C.; Simmons, R.A.; Aghai, Z.H.; Cook, A.; Greenspan, J.S.; DeMauro, S.B. Adverse effects of small for gestational age differ by gestational week among very preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F192–F198. [Google Scholar] [CrossRef]
- He, H.; Miao, H.; Liang, Z.; Zhang, Y.; Jiang, W.; Deng, Z.; Tang, J.; Liu, G.; Luo, X. Prevalence of small for gestational age infants in 21 cities in China, 2014–2019. Sci. Rep. 2021, 11, 7500. [Google Scholar] [CrossRef]
- Lee, A.C.; Katz, J.; Blencowe, H.; Cousens, S.; Kozuki, N.; Vogel, J.P.; Adair, L.; Baqui, A.H.; Bhutta, Z.A.; Caulfield, L.E.; et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob. Health 2013, 1, e26–e36, Erratum in Lancet Glob. Health 2013, 1, e76. [Google Scholar] [CrossRef] [Green Version]
- Magnus, P. Causes of variation in birth weight: A study of offspring of twins. Clin. Genet. 1984, 25, 15–24. [Google Scholar] [CrossRef]
- Järvelin, M.R.; Elliott, P.; Kleinschmidt, I.; Martuzzi, M.; Grundy, C.; Hartikainen, A.L.; Rantakallio, P. Ecological and individual predictors of birthweight in a northern Finland birth cohort 1986. Paediatr. Perinat. Epidemiol. 1997, 11, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Workalemahu, T.; Grantz, K.L.; Grewal, J.; Zhang, C.; Louis, G.M.B.; Tekola-Ayele, F. Genetic and Environmental Influences on Fetal Growth Vary during Sensitive Periods in Pregnancy. Sci. Rep. 2018, 8, 7274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowden, A.L. The insulin-like growth factors and feto-placental growth. Placenta 2003, 24, 803–812. [Google Scholar] [CrossRef]
- White, V.; Jawerbaum, A.; Mazzucco, M.B.; Gauster, M.; Desoye, G.; Hiden, U. IGF2 stimulates fetal growth in a sex- and organ-dependent manner. Pediatr. Res. 2018, 83, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, V.E.; Smith, R.; Giles, W.B.; Clifton, V.L. Endocrine regulation of human fetal growth: The role of the mother, placenta, and fetus. Endocr. Rev. 2006, 27, 141–169. [Google Scholar] [CrossRef]
- Salafia, C.M.; Zhang, J.; Miller, R.K.; Charles, A.K.; Shrout, P.; Sun, W. Placental growth patterns affect birth weight for given placental weight. Birth Defects Res. Part A Clin. Mol. Teratol. 2007, 79, 281–288. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef] [Green Version]
- Chellakooty, M.; Vangsgaard, K.; Larsen, T.; Scheike, T.; Falck-Larsen, J.; Legarth, J.; Andersson, A.M.; Main, K.M.; Skakkebaek, N.E.; Juul, A. A longitudinal study of intrauterine growth and the placental growth hormone (GH)-insulin-like growth factor I axis in maternal circulation: Association between placental GH and fetal growth. J. Clin. Endocrinol. Metab. 2004, 89, 384–391. [Google Scholar] [CrossRef] [Green Version]
- Benton, S.J.; McCowan, L.M.; Heazell, A.E.; Grynspan, D.; Hutcheon, J.A.; Senger, C.; Burke, O.; Chan, Y.; Harding, J.E.; Yockell-Lelièvre, J.; et al. Placental growth factor as a marker of fetal growth restriction caused by placental dysfunction. Placenta 2016, 42, 1–8. [Google Scholar] [CrossRef]
- Chisholm, K.M.; Folkins, A.K. Placental and Clinical Characteristics of Term Small-for-Gestational-Age Neonates: A Case-Control Study. Pediatr. Dev. Pathol. 2016, 19, 37–46. [Google Scholar] [CrossRef]
- Tachibana, M.; Nakayama, M.; Ida, S.; Kitajima, H.; Mitsuda, N.; Ozono, K.; Miyoshi, Y. Pathological examination of the placenta in small for gestational age (SGA) children with or without postnatal catch-up growth. J. Matern. Fetal Neonatal Med. 2016, 29, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Svensson, K.; Tanner, E.; Gennings, C.; Lindh, C.; Kiviranta, H.; Wikström, S.; Bornehag, C.G. Prenatal exposures to mixtures of endocrine disrupting chemicals and children’s weight trajectory up to age 5.5 in the SELMA study. Sci. Rep. 2021, 11, 11036. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.O.; Armitage, J.M.; Bruton, T.A.; Dassuncao, C.; Heiger-Bernays, W.; Hu, X.C.; Kärrman, A.; Kelly, B.; Ng, C.; Robuck, A.; et al. PFAS Exposure Pathways for Humans and Wildlife: A Synthesis of Current Knowledge and Key Gaps in Understanding. Environ. Toxicol. Chem. 2021, 40, 631–657. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.C.; et al. Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J. 2020, 18, e06223. [Google Scholar] [CrossRef] [PubMed]
- Poothong, S.; Papadopoulou, E.; Padilla-Sánchez, J.A.; Thomsen, C.; Haug, L.S. Multiple pathways of human exposure to poly- and perfluoroalkyl substances (PFASs): From external exposure to human blood. Environ. Int. 2020, 134, 105244. [Google Scholar] [CrossRef]
- US EPA (United States Environmental Protection Agency). EPA’s Per- and Polyfluoroalkyl Substances (PFAS) Action Plan. 2019. Available online: https://www.epa.gov/sites/default/files/2019-02/documents/pfas_action_plan_021319_508compliant_1.pdf (accessed on 6 November 2022).
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological Profile for Perfluoroalkyls; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2021. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp200-c2.pdf (accessed on 6 November 2022).
- Bach, C.C.; Bech, B.H.; Brix, N.; Nohr, E.A.; Bonde, J.P.; Henriksen, T.B. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: A systematic review. Crit. Rev. Toxicol. 2015, 45, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Negri, E.; Metruccio, F.; Guercio, V.; Tosti, L.; Benfenati, E.; Bonzi, R.; La Vecchia, C.; Moretto, A. Exposure to PFOA and PFOS and fetal growth: A critical merging of toxicological and epidemiological data. Crit. Rev. Toxicol. 2017, 47, 482–508, Erratum in Crit. Rev. Toxicol. 2017, 47, i. [Google Scholar] [CrossRef]
- Dzierlenga, M.W.; Crawford, L.; Longnecker, M.P. Birth weight and perfluorooctane sulfonic acid: A random-effects meta-regression analysis. Environ. Epidemiol. 2020, 4, e095. [Google Scholar] [CrossRef]
- Cao, T.; Qu, A.; Li, Z.; Wang, W.; Liu, R.; Wang, X.; Nie, Y.; Sun, S.; Zhang, X.; Liu, X. The relationship between maternal perfluoroalkylated substances exposure and low birth weight of offspring: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 2021, 28, 67053–67065. [Google Scholar] [CrossRef]
- Lee, Y.J.; Jung, H.W.; Kim, H.Y.; Choi, Y.-J.; Lee, Y.A. Early-Life Exposure to Per- and Poly-Fluorinated Alkyl Substances and Growth, Adiposity, and Puberty in Children: A Systematic Review. Front. Endocrinol. 2021, 12, 683297. [Google Scholar] [CrossRef]
- Fan, X.; Tang, S.; Wang, Y.; Fan, W.; Ben, Y.; Naidu, R.; Dong, Z. Global Exposure to Per- and Polyfluoroalkyl Substances and Associated Burden of Low Birthweight. Environ. Sci. Technol. 2022, 56, 4282–4294. [Google Scholar] [CrossRef] [PubMed]
- Rokoff, L.B.; Rifas-Shiman, S.L.; Coull, B.A.; Cardenas, A.; Calafat, A.M.; Ye, X.; Gryparis, A.; Schwartz, J.; Sagiv, S.K.; Gold, D.R.; et al. Cumulative exposure to environmental pollutants during early pregnancy and reduced fetal growth: The Project Viva cohort. Environ. Health 2018, 17, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.J.; Barr, D.B.; Ryan, P.B.; Panuwet, P.; Smarr, M.M.; Liu, K.; Kannan, K.; Yakimavets, V.; Tan, Y.; Ly, V.; et al. Per- and polyfluoroalkyl substance (PFAS) exposure, maternal metabolomic perturbation, and fetal growth in African American women: A meet-in-the-middle approach. Environ. Int. 2022, 158, 106964. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard-Olesen, C.; Bach, C.C.; Long, M.; Wielsøe, M.; Bech, B.H.; Henriksen, T.B.; Olsen, J.; Bonefeld-Jørgensen, E.C. Associations of Fetal Growth Outcomes with Measures of the Combined Xenoestrogenic Activity of Maternal Serum Perfluorinated Alkyl Acids in Danish Pregnant Women. Environ. Health Perspect. 2019, 127, 17006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bommarito, P.A.; Ferguson, K.K.; Meeker, J.D.; McElrath, T.F.; Cantonwine, D.E. Maternal Levels of Perfluoroalkyl Substances (PFAS) during Early Pregnancy in Relation to Preeclampsia Subtypes and Biomarkers of Preeclampsia Risk. Environ. Health Perspect. 2021, 129, 107004. [Google Scholar] [CrossRef]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef] [Green Version]
- Talia, C.; Connolly, L.; Fowler, P.A. The insulin-like growth factor system: A target for endocrine disruptors? Environ. Int. 2021, 147, 106311. [Google Scholar] [CrossRef]
- Forsthuber, M.; Widhalm, R.; Granitzer, S.; Kaiser, A.M.; Moshammer, H.; Hengstschläger, M.; Dolznig, H.; Gundacker, C. Perfluorooctane sulfonic acid (PFOS) inhibits vessel formation in a human 3D co-culture angiogenesis model (NCFs/HUVECs). Environ. Pollut. 2022, 293, 118543. [Google Scholar] [CrossRef]
- Fei, C.; McLaughlin, J.K.; Tarone, R.E.; Olsen, J. Fetal growth indicators and perfluorinated chemicals: A study in the Danish National Birth Cohort. Am. J. Epidemiol. 2008, 168, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Deng, Y.; Song, Z.; Xie, Y.; Gong, L.; Chen, Y.; Kuang, H. Gestational Perfluorooctanoic Acid Exposure Inhibits Placental Development by Dysregulation of Labyrinth Vessels and uNK Cells and Apoptosis in Mice. Front. Physiol. 2020, 11, 51. [Google Scholar] [CrossRef]
- Blake, B.E.; Cope, H.A.; Hall, S.M.; Keys, R.D.; Mahler, B.W.; McCord, J.; Scott, B.; Stapleton, H.M.; Strynar, M.J.; Elmore, S.A.; et al. Evaluation of Maternal, Embryo, and Placental Effects in CD-1 Mice following Gestational Exposure to Perfluorooctanoic Acid (PFOA) or Hexafluoropropylene Oxide Dimer Acid (HFPO-DA or GenX). Environ. Health Perspect. 2020, 128, 27006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, W.S.; Li, W.J.; Liu, C.; Wang, Y.; Sun, K. Reduction of progesterone, estradiol and hCG secretion by perfluorooctane sulfonate via induction of apoptosis in human placental syncytiotrophoblasts. Placenta 2015, 36, 575–580. [Google Scholar] [CrossRef] [PubMed]
- HBM4EU. Available online: https://www.hbm4eu.eu (accessed on 6 November 2022).
- AOP-helpFinder. Available online: http://aop-helpfinder.u-paris-sciences.fr (accessed on 6 November 2022).
- Carvaillo, J.C.; Barouki, R.; Coumoul, X.; Audouze, K. Linking Bisphenol S to Adverse Outcome Pathways Using a Combined Text Mining and Systems Biology Approach. Environ. Health Perspect. 2019, 127, 47005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rugard, M.; Coumoul, X.; Carvaillo, J.C.; Barouki, R.; Audouze, K. Deciphering Adverse Outcome Pathway Network Linked to Bisphenol F Using Text Mining and Systems Toxicology Approaches. Toxicol. Sci. 2020, 173, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benoit, L.; Jornod, F.; Zgheib, E.; Tomkiewicz, C.; Koual, M.; Coustillet, T.; Barouki, R.; Audouze, K.; Vinken, M.; Coumoul, X. Adverse outcome pathway from activation of the AhR to breast cancer-related death. Environ. Int. 2022, 165, 107323. [Google Scholar] [CrossRef]
- Jaylet, T.; Quintens, R.; Benotmane, M.A.; Luukkonen, J.; Tanaka, I.B., 3rd; Ibanez, C.; Durand, C.; Sachana, M.; Azimzadeh, O.; Adam-Guillermin, C.; et al. Development of an adverse outcome pathway for radiation-induced microcephaly via expert consultation and machine learning. Int. J. Radiat. Biol. 2022, 1–11. [Google Scholar] [CrossRef]
- Kaiser, A.M.; Zare Jeddi, M.; Uhl, M.; Jornod, F.; Fernandez, M.F.; Audouze, K. Characterization of Potential Adverse Outcome Pathways Related to Metabolic Outcomes and Exposure to Per- and Polyfluoroalkyl Substances Using Artificial Intelligence. Toxics 2022, 10, 449. [Google Scholar] [CrossRef]
- Jornod, F.; Jaylet, T.; Blaha, L.; Sarigiannis, D.; Tamisier, L.; Audouze, K. AOP-helpFinder webserver: A tool for comprehensive analysis of the literature to support adverse outcome pathways development. Bioinformatics 2021, 38, 1173–1175. [Google Scholar] [CrossRef]
- Kim, H.M.; Long, N.P.; Yoon, S.J.; Anh, N.H.; Kim, S.J.; Park, J.H.; Kwon, S.W. Omics approach reveals perturbation of metabolism and phenotype in Caenorhabditis elegans triggered by perfluorinated compounds. Sci. Total Environ. 2020, 703, 135500. [Google Scholar] [CrossRef]
- Kim, J.H.; Barbagallo, B.; Annunziato, K.; Farias-Pereira, R.; Doherty, J.J.; Lee, J.; Zina, J.; Tindal, C.; McVey, C.; Aresco, R.; et al. Maternal preconception PFOS exposure of Drosophila melanogaster alters reproductive capacity, development, morphology and nutrient regulation. Food Chem. Toxicol. 2021, 151, 112153. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Wong, C.K.; Oger, C.; Durand, T.; Galano, J.-M.; Lee, J.C.-Y. Prenatal exposure to the contaminant perfluorooctane sulfonate elevates lipid peroxidation during mouse fetal development but not in the pregnant dam. Free Radic. Res. 2015, 49, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Quan, X.-J.; Chen, G.; Hong, J.-W.; Wang, Q.; Xu, L.-L.; Wang, B.-H.; Yu, Z.-H.; Yu, H.-M. PFOS-induced placental cell growth inhibition is partially mediated by lncRNA H19 through interacting with miR-19a and miR-19b. Chemosphere 2020, 261, 127640. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, Z.; Gao, P.; Yin, D. Multigenerational effects of perfluorooctanoic acid on lipid metabolism of Caenorhabditis elegans and its potential mechanism. Sci. Total Environ. 2020, 703, 134762. [Google Scholar] [CrossRef]
- Ortiz-Villanueva, E.; Jaumot, J.; Martínez, R.; Navarro-Martín, L.; Piña, B.; Tauler, R. Assessment of endocrine disruptors effects on zebrafish (Danio rerio) embryos by untargeted LC-HRMS metabolomic analysis. Sci. Total Environ. 2018, 635, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kim, W.-S.; Choi, B.; Kwak, I.-S. Expression Levels of the Immune-Related p38 Mitogen-Activated Protein Kinase Transcript in Response to Environmental Pollutants on Macrophthalmus japonicus Crab. Genes 2020, 11, 958. [Google Scholar] [CrossRef]
- Qiu, L.; Qian, Y.; Liu, Z.; Wang, C.; Qu, J.; Wang, X.; Wang, S. Perfluorooctane sulfonate (PFOS) disrupts blood-testis barrier by down-regulating junction proteins via p38 MAPK/ATF2/MMP9 signaling pathway. Toxicology 2016, 373, 1–12. [Google Scholar] [CrossRef]
- Seyoum, A.; Pradhan, A.; Jass, J.; Olsson, P.E. Perfluorinated alkyl substances impede growth, reproduction, lipid metabolism and lifespan in Daphnia magna. Sci. Total Environ. 2020, 737, 139682. [Google Scholar] [CrossRef]
- Wan, H.T.; Wong, A.-Y.; Feng, S.; Wong, C.-K. Effects of In Utero Exposure to Perfluorooctane Sulfonate on Placental Functions. Environ. Sci. Technol. 2020, 54, 16050–16061. [Google Scholar] [CrossRef]
- Wang, B.; Li, D.; Yuan, Z.; Zhang, Y.; Ma, X.; Lv, Z.; Xiao, Y.; Zhang, J. Evaluation of joint effects of perfluorooctane sulfonate and wood vinegar on planarians, Dugesia japonica. Environ. Sci. Pollut. Res. Int. 2020, 27, 18089–18098. [Google Scholar] [CrossRef]
- Xia, X.; Yu, R.; Li, M.; Liu, L.; Zhang, K.; Wang, Y.; Li, B.; Zhang, L.; Song, G.; Zheng, X.; et al. Molecular cloning and characterization of two genes encoding peroxiredoxins from freshwater bivalve Anodonta woodiana: Antioxidative effect and immune defense. Fish Shellfish Immunol. 2018, 82, 476–491. [Google Scholar] [CrossRef]
- Yue, Y.; Li, S.; Qian, Z.; Pereira, R.F.; Lee, J.; Doherty, J.J.; Zhang, Z.; Peng, Y.; Clark, J.M.; Timme-Laragy, A.R.; et al. Perfluorooctanesulfonic acid (PFOS) and perfluorobutanesulfonic acid (PFBS) impaired reproduction and altered offspring physiological functions in Caenorhabditis elegans. Food Chem. Toxicol. 2020, 145, 111695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, W.; Chen, H.; Tian, F.; Cai, W. Transcriptome analysis of acute exposure of the Manila clam, Ruditapes philippinarum to perfluorooctane sulfonate (PFOS). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 231, 108736. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Sun, J.; Zhang, Y. Perfluorooctanoic acid impaired glucose homeostasis through affecting adipose AKT pathway. Cytotechnology 2018, 70, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Guruge, K.S.; Yeung, L.W.; Yamanaka, N.; Miyazaki, S.; Lam, P.K.; Giesy, J.P.; Jones, P.D.; Yamashita, N. Gene expression profiles in rat liver treated with perfluorooctanoic acid (PFOA). Toxicol. Sci. 2006, 89, 93–107. [Google Scholar] [CrossRef]
- Li, D.; Zhang, L.; Zhang, Y.; Guan, S.; Gong, X.; Wang, X. Maternal exposure to perfluorooctanoic acid (PFOA) causes liver toxicity through PPAR-α pathway and lowered histone acetylation in female offspring mice. Environ. Sci. Pollut. Res. Int. 2019, 26, 18866–18875. [Google Scholar] [CrossRef]
- Li, F.; Yu, Y.; Guo, M.; Lin, Y.; Jiang, Y.; Qu, M.; Sun, X.; Li, Z.; Zhai, Y.; Tan, Z. Integrated analysis of physiological, transcriptomics and metabolomics provides insights into detoxication disruption of PFOA exposure in Mytilus edulis. Ecotoxicol. Environ. Saf. 2021, 214, 112081. [Google Scholar] [CrossRef]
- Liu, W.; Yang, B.; Wu, L.; Zou, W.; Pan, X.; Zou, T.; Liu, F.; Xia, L.; Wang, X.; Zhang, D. Involvement of NRF2 in Perfluorooctanoic Acid-Induced Testicular Damage in Male Mice. Biol. Reprod. 2015, 93, 41. [Google Scholar] [CrossRef]
- Liu, R.C.M.; Hurtt, M.E.; Cook, J.C.; Biegel, L.B. Effect of the peroxisome proliferator, ammonium perfluorooctanoate (C8), on hepatic aromatase activity in adult male Crl:CD BR (CD) rats. Fundam. Appl. Toxicol. 1996, 30, 220–228. [Google Scholar] [CrossRef]
- Salimi, A.; Nikoosiar Jahromi, M.; Pourahmad, J. Maternal exposure causes mitochondrial dysfunction in brain, liver, and heart of mouse fetus: An explanation for perfluorooctanoic acid induced abortion and developmental toxicity. Environ. Toxicol. 2019, 34, 878–885. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Liu, Y.; Zhang, H.; Dai, J. Disturbance of perfluorooctanoic acid on development and behavior in Drosophila larvae. Environ. Toxicol. Chem. 2010, 29, 2117–2122. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, H.; Zheng, F.; Sheng, N.; Guo, X.; Dai, J. Perfluorooctanoic acid exposure for 28 days affects glucose homeostasis and induces insulin hypersensitivity in mice. Sci. Rep. 2015, 5, 11029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.-H.; Glover, K.P.; Han, X. Characterization of cellular uptake of perfluorooctanoate via organic anion-transporting polypeptide 1A2, organic anion transporter 4, and urate transporter 1 for their potential roles in mediating human renal reabsorption of perfluorocarboxylates. Toxicol. Sci. 2010, 117, 294–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, W.A.; Guyton, K.Z.; Martin, M.T.; Reif, D.M.; Rusyn, I. Use of high-throughput in vitro toxicity screening data in cancer hazard evaluations by IARC Monograph Working Groups. Altex 2018, 35, 51–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorrochategui, E.; Pérez-Albaladejo, E.; Casas, J.; Lacorte, S.; Porte, C. Perfluorinated chemicals: Differential toxicity, inhibition of aromatase activity and alteration of cellular lipids in human placental cells. Toxicol. Appl. Pharmacol. 2014, 277, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Gorrochategui, E.; Lacorte, S.; Tauler, R.; Martin, F.L. Perfluoroalkylated Substance Effects in Xenopus laevis A6 Kidney Epithelial Cells Determined by ATR-FTIR Spectroscopy and Chemometric Analysis. Chem. Res. Toxicol. 2016, 29, 924–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reistad, T.; Fonnum, F.; Mariussen, E. Perfluoroalkylated compounds induce cell death and formation of reactive oxygen species in cultured cerebellar granule cells. Toxicol. Lett. 2013, 218, 56–60. [Google Scholar] [CrossRef]
- Sun, P.; Nie, X.; Chen, X.; Yin, L.; Luo, J.; Sun, L.; Wan, C.; Jiang, S. Nrf2 Signaling Elicits a Neuroprotective Role against PFOS-mediated Oxidative Damage and Apoptosis. Neurochem. Res. 2018, 43, 2446–2459. [Google Scholar] [CrossRef]
- Sun, P.; Gu, L.; Luo, J.; Qin, Y.; Sun, L.; Jiang, S. ROS-mediated JNK pathway critically contributes to PFOS-triggered apoptosis in SH-SY5Y cells. Neurotoxicol. Teratol. 2019, 75, 106821. [Google Scholar] [CrossRef]
- Tang, L.-L.; Wang, J.-D.; Xu, T.-T.; Zhao, Z.; Zheng, J.-J.; Ge, R.-S.; Zhu, D.-Y. Mitochondrial toxicity of perfluorooctane sulfonate in mouse embryonic stem cell-derived cardiomyocytes. Toxicology 2017, 382, 108–116. [Google Scholar] [CrossRef]
- Wang, C.; Nie, X.; Zhang, Y.; Li, T.; Mao, J.; Liu, X.; Gu, Y.; Shi, J.; Xiao, J.; Wan, C.; et al. Reactive oxygen species mediate nitric oxide production through ERK/JNK MAPK signaling in HAPI microglia after PFOS exposure. Toxicol. Appl. Pharmacol. 2015, 288, 143–151. [Google Scholar] [CrossRef]
- Wei, Y.; Shi, X.; Zhang, H.; Wang, J.; Zhou, B.; Dai, J. Combined effects of polyfluorinated and perfluorinated compounds on primary cultured hepatocytes from rare minnow (Gobiocypris rarus) using toxicogenomic analysis. Aquat. Toxicol. 2009, 95, 27–36. [Google Scholar] [CrossRef]
- Xu, J.; Shimpi, P.; Armstrong, L.; Salter, D.; Slitt, A.L. PFOS induces adipogenesis and glucose uptake in association with activation of Nrf2 signaling pathway. Toxicol. Appl. Pharmacol. 2016, 290, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarei, M.H.; Shirazi, S.F.H.; Aghvami, M.; Pourahmad, J. Perfluorooctanesulfonate (PFOS) Induces Apoptosis Signaling and Proteolysis in Human Lymphocytes through ROS Mediated Mitochondrial Dysfunction and Lysosomal Membrane Labialization. Iran. J. Pharm. Res. 2018, 17, 995–1007. [Google Scholar] [PubMed]
- Lu, Y.; Pan, Y.; Sheng, N.; Zhao, A.Z.; Dai, J. Perfluorooctanoic acid exposure alters polyunsaturated fatty acid composition, induces oxidative stress and activates the AKT/AMPK pathway in mouse epididymis. Chemosphere 2016, 158, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, V.; Tehrani, K.H.; Hashemzaei, M.; Tabrizian, K.; Shahraki, J.; Hosseini, M.J. Mechanistic approach for the toxic effects of perfluorooctanoic acid on isolated rat liver and brain mitochondria. Hum. Exp. Toxicol. 2015, 34, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.S.; Choi, E.M.; Kim, Y.J.; Hong, S.M.; Park, S.Y.; Rhee, S.Y.; Oh, S.; Kim, S.W.; Pak, Y.K.; Choe, W.; et al. Perfluorooctanoic acid induces oxidative damage and mitochondrial dysfunction in pancreatic β-cells. Mol. Med. Rep. 2017, 15, 3871–3878. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Lu, X.; Chen, F.; Ye, X.; Zhou, D.; Yuan, J.; He, J.; Chen, B.; Shan, X.; Jiang, J.; et al. Effects of Perfluorooctanoic Acid on the Associated Genes Expression of Autophagy Signaling Pathway of Carassius auratus Lymphocytes in vitro. Front. Physiol. 2018, 9, 1748. [Google Scholar] [CrossRef]
- Tian, J.; Hong, Y.; Li, Z.; Yang, Z.; Lei, B.; Liu, J.; Cai, Z. Immunometabolism-modulation and immunotoxicity evaluation of perfluorooctanoic acid in macrophage. Ecotoxicol. Environ. Saf. 2021, 215, 112128. [Google Scholar] [CrossRef]
- Lee, Y.J.; Choi, S.-Y.; Yang, J.-H. PFHxS induces apoptosis of neuronal cells via ERK1/2-mediated pathway. Chemosphere 2014, 94, 121–127. [Google Scholar] [CrossRef]
- Lee, Y.J.; Choi, S.-Y.; Yang, J.-H. NMDA receptor-mediated ERK 1/2 pathway is involved in PFHxS-induced apoptosis of PC12 cells. Sci. Total Environ. 2014, 491–492, 227–234. [Google Scholar] [CrossRef]
- Dong, T.; Peng, Y.; Zhong, N.; Liu, F.; Zhang, H.; Xu, M.; Liu, R.; Han, M.; Tian, X.; Jia, J.; et al. Perfluorodecanoic acid (PFDA) promotes gastric cell proliferation via sPLA2-IIA. Oncotarget 2017, 8, 50911–50920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleszczyński, K.; Składanowski, A.C. Mechanism of cytotoxic action of perfluorinated acids. III. Disturbance in Ca2+ homeostasis. Toxicol. Appl. Pharmacol. 2011, 251, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, T.; Lv, C.; Niu, Q.; Zong, W.; Tang, J.; Liu, R. Perfluorodecanoic acid-induced oxidative stress and DNA damage investigated at the cellular and molecular levels. Ecotoxicol. Environ. Saf. 2019, 185, 109699. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-H. Perfluorooctanoic acid induces peroxisomal fatty acid oxidation and cytokine expression in the liver of male Japanese medaka (Oryzias latipes). Chemosphere 2010, 81, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, L.S.; Bonefeld-Jørgensen, E.C. Perfluorinated compounds affect the function of sex hormone receptors. Environ. Sci. Pollut. Res. Int. 2013, 20, 8031–8044. [Google Scholar] [CrossRef]
- Kang, J.S.; Choi, J.-S.; Park, J.-W. Transcriptional changes in steroidogenesis by perfluoroalkyl acids (PFOA and PFOS) regulate the synthesis of sex hormones in H295R cells. Chemosphere 2016, 155, 436–443. [Google Scholar] [CrossRef]
- Behr, A.C.; Lichtenstein, D.; Braeuning, A.; Lampen, A.; Buhrke, T. Perfluoroalkylated substances (PFAS) affect neither estrogen and androgen receptor activity nor steroidogenesis in human cells in vitro. Toxicol. Lett. 2018, 291, 51–60. [Google Scholar] [CrossRef]
- Bao, M.; Huang, W.; Au, W.W.; Zheng, S.; Liu, C.; Huang, Y.; Wu, K. Exposure to perfluorooctane sulfonate based on circadian rhythm changes the fecundity and expression of certain genes on the hypothalamic-pituitary-gonadal-liver axis of female zebrafish. Toxicol. Appl. Pharmacol. 2019, 381, 114715. [Google Scholar] [CrossRef]
- Bao, M.; Zheng, S.; Liu, C.; Huang, W.; Xiao, J.; Wu, K. Perfluorooctane sulfonate exposure alters sexual behaviors and transcriptions of genes in hypothalamic-pituitary-gonadal-liver axis of male zebrafish (Danio rerio). Environ. Pollut. 2020, 267, 115585. [Google Scholar] [CrossRef]
- Benninghoff, A.D.; Bisson, W.H.; Koch, D.C.; Ehresman, D.J.; Kolluri, S.K.; Williams, D.E. Estrogen-like activity of perfluoroalkyl acids in vivo and interaction with human and rainbow trout estrogen receptors in vitro. Toxicol. Sci. 2011, 120, 42–58. [Google Scholar] [CrossRef]
- Biegel, L.B.; Liu, R.C.; Hurtt, M.E.; Cook, J.C. Effects of ammonium perfluorooctanoate on Leydig cell function: In vitro, in vivo, and ex vivo studies. Toxicol. Appl. Pharmacol. 1995, 134, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Ge, X.; Wang, D.; Wang, T.; Zhang, L.; Tanguay, R.L.; Simonich, M.; Huang, C.; Dong, Q. Chronic perfluorooctanesulphonic acid (PFOS) exposure produces estrogenic effects in zebrafish. Environ. Pollut. 2016, 218, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Hu, J.; Huang, H.; Qin, Y.; Han, X.; Wu, D.; Song, L.; Xia, Y.; Wang, X. Perfluorooctane sulfonate (PFOS) affects hormone receptor activity, steroidogenesis, and expression of endocrine-related genes in vitro and in vivo. Environ. Toxicol. Chem. 2013, 32, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.-H.; Lu, C.-C.; Xu, C.; Chen, G.; Qiu, L.-L.; Jiang, J.-K.; Ben, S.; Wang, Y.-B.; Gu, A.-H.; Wang, X.-R. Perfluorooctane sulfonate-induced testicular toxicity and differential testicular expression of estrogen receptor in male mice. Environ. Toxicol. Pharmacol. 2016, 45, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Qu, K.; Luan, F.; Liu, Y.; Zhu, Y.; Yuan, Y.; Li, H.; Zhang, H.; Hai, Y.; Zhao, C. Binding specificities of estrogen receptor with perfluorinated compounds: A cross species comparison. Environ. Int. 2020, 134, 105284. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, H.; Dong, T.; Huang, J.; Li, T.; Ren, H.; Wang, X.; Qu, J.; Wang, S. Perfluorooctane sulfonate (PFOS) disrupts testosterone biosynthesis via CREB/CRTC2/StAR signaling pathway in Leydig cells. Toxicology 2021, 449, 152663. [Google Scholar] [CrossRef]
- Rodríguez-Jorquera, I.A.; Colli-Dula, R.C.; Kroll, K.; Jayasinghe, B.S.; Parachu Marco, M.V.; Silva-Sanchez, C.; Toor, G.S.; Denslow, N.D. Blood Transcriptomics Analysis of Fish Exposed to Perfluoro Alkyls Substances: Assessment of a Non-Lethal Sampling Technique for Advancing Aquatic Toxicology Research. Environ. Sci. Technol. 2019, 53, 1441–1452. [Google Scholar] [CrossRef]
- Rosen, M.B.; Das, K.P.; Rooney, J.; Abbott, B.; Lau, C.; Corton, J.C. PPARα-independent transcriptional targets of perfluoroalkyl acids revealed by transcript profiling. Toxicology 2017, 387, 95–107. [Google Scholar] [CrossRef]
- Xin, Y.; Wan, B.; Yu, B.; Fan, Y.; Chen, D.; Guo, L.-H. Chlorinated Polyfluoroalkylether Sulfonic Acids Exhibit Stronger Estrogenic Effects than Perfluorooctane Sulfonate by Activating Nuclear Estrogen Receptor Pathways. Environ. Sci. Technol. 2020, 54, 3455–3464. [Google Scholar] [CrossRef]
- Xu, C.; Jiang, Z.-Y.; Liu, Q.; Liu, H.; Gu, A. Estrogen receptor beta mediates hepatotoxicity induced by perfluorooctane sulfonate in mouse. Environ. Sci. Pollut. Res. Int. 2017, 24, 13414–13423. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, H.; Chen, P.; Chen, X.; Sun, C.; Ge, R.-S.; Su, Z.; Ye, L. Effects of gestational Perfluorooctane Sulfonate exposure on the developments of fetal and adult Leydig cells in F1 males. Environ. Pollut. 2020, 262, 114241. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, L.; Liu, J.; Li, H.; Zhang, C.; Han, P.; Zhang, Y.; Yuan, X.; Ge, R.-S.; Chu, Y. Exposure to perfluorooctane sulfonate in utero reduces testosterone production in rat fetal Leydig cells. PLoS ONE 2014, 9, e78888. [Google Scholar] [CrossRef] [Green Version]
- Zhong, S.-Q.; Chen, Z.-X.; Kong, M.-L.; Xie, Y.-Q.; Zhou, Y.; Qin, X.-D.; Paul, G.; Zeng, X.-W.; Dong, G.-H. Testosterone-Mediated Endocrine Function and TH1/TH2 Cytokine Balance after Prenatal Exposure to Perfluorooctane Sulfonate: By Sex Status. Int. J. Mol. Sci. 2016, 17, 1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Zhang, H.; Gao, J.; Li, Z.; Bao, S.; Chen, X.; Wang, Y.; Ge, R.; Ye, L. Effects of perfluorooctanoic acid on stem Leydig cell functions in the rat. Environ. Pollut. 2019, 250, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Dai, J.; Liu, M.; Wang, J.; Xu, M.; Zha, J.; Wang, Z. Estrogen-like properties of perfluorooctanoic acid as revealed by expressing hepatic estrogen-responsive genes in rare minnows (Gobiocypris rarus). Environ. Toxicol. Chem. 2007, 26, 2440–2447. [Google Scholar] [CrossRef]
- Xin, Y.; Ren, X.-M.; Wan, B.; Guo, L.-H. Comparative In Vitro and In Vivo Evaluation of the Estrogenic Effect of Hexafluoropropylene Oxide Homologues. Environ. Sci. Technol. 2019, 53, 8371–8380. [Google Scholar] [CrossRef]
- Yao, P.-L.; Ehresman, D.J.; Rae, J.M.; Chang, S.-C.; Frame, S.R.; Butenhoff, J.L.; Kennedy, G.L.; Peters, J.M. Comparative in vivo and in vitro analysis of possible estrogenic effects of perfluorooctanoic acid. Toxicology 2014, 326, 62–73. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, Y.S.; Haslam, S.Z.; Yang, C. Perfluorooctanoic acid effects on steroid hormone and growth factor levels mediate stimulation of peripubertal mammary gland development in C57BL/6 mice. Toxicol. Sci. 2010, 115, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Shi, Z.; Fang, X.; Xu, M.; Dai, J. Perfluorononanoic acid induces apoptosis involving the Fas death receptor signaling pathway in rat testis. Toxicol. Lett. 2009, 190, 224–230. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K. Prepubertal exposure to perfluorononanoic acid interferes with spermatogenesis and steroidogenesis in male mice. Ecotoxicol. Environ. Saf. 2019, 170, 590–599. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K. Acute exposure to perfluorononanoic acid in prepubertal mice: Effect on germ cell dynamics and an insight into the possible mechanisms of its inhibitory action on testicular functions. Ecotoxicol. Environ. Saf. 2019, 183, 109499. [Google Scholar] [CrossRef] [PubMed]
- Gogola, J.; Hoffmann, M.; Nimpsz, S.; Ptak, A. Disruption of 17β-estradiol secretion by persistent organic pollutants present in human follicular fluid is dependent on the potential of ovarian granulosa tumor cell lines to metabolize estrogen. Mol. Cell. Endocrinol. 2020, 503, 110698. [Google Scholar] [CrossRef] [PubMed]
- Halsne, R.; Tandberg, J.I.; Lobert, V.H.; Østby, G.C.; Thoen, E.; Ropstad, E.; Verhaegen, S. Effects of perfluorinated alkyl acids on cellular responses of MCF-10A mammary epithelial cells in monolayers and on acini formation in vitro. Toxicol. Lett. 2016, 259, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Maras, M.; Vanparys, C.; Muylle, F.; Robbens, J.; Berger, U.; Barber, J.L.; Blust, R.; De Coen, W. Estrogen-like properties of fluorotelomer alcohols as revealed by mcf-7 breast cancer cell proliferation. Environ. Health Perspect. 2006, 114, 100–105. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Cao, H.; Feng, H.; Xue, Q.; Zhang, A.; Fu, J. Evaluation of the Estrogenic/Antiestrogenic Activities of Perfluoroalkyl Substances and Their Interactions with the Human Estrogen Receptor by Combining In Vitro Assays and In Silico Modeling. Environ. Sci. Technol. 2020, 54, 14514–14524. [Google Scholar] [CrossRef]
- Ishibashi, H.; Yamauchi, R.; Matsuoka, M.; Kim, J.-W.; Hirano, M.; Yamaguchi, A.; Tominaga, N.; Arizono, K. Fluorotelomer alcohols induce hepatic vitellogenin through activation of the estrogen receptor in male medaka (Oryzias latipes). Chemosphere 2008, 71, 1853–1859. [Google Scholar] [CrossRef]
- Buhrke, T.; Krüger, E.; Pevny, S.; Rößler, M.; Bitter, K.; Lampen, A. Perfluorooctanoic acid (PFOA) affects distinct molecular signalling pathways in human primary hepatocytes. Toxicology 2015, 333, 53–62. [Google Scholar] [CrossRef]
- Rosenmai, A.K.; Nielsen, F.K.; Pedersen, M.; Hadrup, N.; Trier, X.; Christensen, J.H.; Vinggaard, A.M. Fluorochemicals used in food packaging inhibit male sex hormone synthesis. Toxicol. Appl. Pharmacol. 2013, 266, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Gogola, J.; Hoffmann, M.; Ptak, A. Persistent endocrine-disrupting chemicals found in human follicular fluid stimulate IGF1 secretion by adult ovarian granulosa cell tumor spheroids and thereby increase proliferation of non-cancer ovarian granulosa cells. Toxicol. In Vitro 2020, 65, 104769. [Google Scholar] [CrossRef]
- Dairkee, S.H.; Luciani-Torres, G.; Moore, D.H.; Jaffee, I.M.; Goodson, W.H., 3rd. A Ternary Mixture of Common Chemicals Perturbs Benign Human Breast Epithelial Cells More Than the Same Chemicals Do Individually. Toxicol. Sci. 2018, 165, 131–144. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, L.; Tian, L.; Wang, N.; Lei, L.; Wang, Y.; Dong, Q.; Huang, C.; Yang, D. Chronic PFOS Exposure Disrupts Thyroid Structure and Function in Zebrafish. Bull. Environ. Contam. Toxicol. 2018, 101, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ji, K.; Lee, S.; Lee, J.; Kim, J.; Kim, S.; Kho, Y.; Choi, K. Perfluorooctane sulfonic acid exposure increases cadmium toxicity in early life stage of zebrafish, Danio rerio. Environ. Toxicol. Chem. 2011, 30, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.-M.; Zhang, Y.-F.; Guo, L.-H.; Qin, Z.-F.; Lv, Q.-Y.; Zhang, L.-Y. Structure-activity relations in binding of perfluoroalkyl compounds to human thyroid hormone T3 receptor. Arch. Toxicol. 2015, 89, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Liu, C.; Wu, G.; Zhou, B. Waterborne exposure to PFOS causes disruption of the hypothalamus-pituitary-thyroid axis in zebrafish larvae. Chemosphere 2009, 77, 1010–1018, Erratum in Chemosphere 2010, 81, 821. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.-G.; Liu, W.; Liu, L.; Jin, Y.-H. Perfluorooctane sulfonate increased hepatic expression of OAPT2 and MRP2 in rats. Arch. Toxicol. 2011, 85, 613–621. [Google Scholar] [CrossRef]
- Chang, S.C.; Thibodeaux, J.R.; Eastvold, M.L.; Ehresman, D.J.; Bjork, J.A.; Froehlich, J.W.; Lau, C.; Singh, R.J.; Wallace, K.B.; Butenhoff, J.L. Thyroid hormone status and pituitary function in adult rats given oral doses of perfluorooctanesulfonate (PFOS). Toxicology 2008, 243, 330–339. [Google Scholar] [CrossRef]
- Deng, M.; Wu, Y.; Xu, C.; Jin, Y.; He, X.; Wan, J.; Yu, X.; Rao, H.; Tu, W. Multiple approaches to assess the effects of F-53B, a Chinese PFOS alternative, on thyroid endocrine disruption at environmentally relevant concentrations. Sci. Total Environ. 2018, 624, 215–224. [Google Scholar] [CrossRef]
- Hong, S.-H.; Lee, S.H.; Yang, J.Y.; Lee, J.H.; Jung, K.K.; Seok, J.H.; Kim, S.-H.; Nam, K.T.; Jeong, J.; Lee, J.K.; et al. Orally Administered 6:2 Chlorinated Polyfluorinated Ether Sulfonate (F-53B) Causes Thyroid Dysfunction in Rats. Toxics 2020, 8, 54. [Google Scholar] [CrossRef]
- Godfrey, A.; Hooser, B.; Abdelmoneim, A.; Sepúlveda, M.S. Sex-specific endocrine-disrupting effects of three halogenated chemicals in Japanese medaka. J. Appl. Toxicol. 2019, 39, 1215–1223. [Google Scholar] [CrossRef]
- Kim, J.; Lee, G.; Lee, Y.-M.; Zoh, K.-D.; Choi, K. Thyroid disrupting effects of perfluoroundecanoic acid and perfluorotridecanoic acid in zebrafish (Danio rerio) and rat pituitary (GH3) cell line. Chemosphere 2021, 262, 128012. [Google Scholar] [CrossRef]
- Conley, J.M.; Lambright, C.S.; Evans, N.; McCord, J.; Strynar, M.J.; Hill, D.; Medlock-Kakaley, E.; Wilson, V.S.; Gray, L.E., Jr. Hexafluoropropylene oxide-dimer acid (HFPO-DA or GenX) alters maternal and fetal glucose and lipid metabolism and produces neonatal mortality, low birthweight, and hepatomegaly in the Sprague-Dawley rat. Environ. Int. 2021, 146, 106204. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Fang, X.; Zhang, H.; Dai, J. The thyroid-disrupting effects of long-term perfluorononanoate exposure on zebrafish (Danio rerio). Ecotoxicology 2011, 20, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ramhøj, L.; Hass, U.; Gilbert, M.E.; Wood, C.; Svingen, T.; Usai, D.; Vinggaard, A.M.; Mandrup, K.; Axelstad, M. Evaluating thyroid hormone disruption: Investigations of long-term neurodevelopmental effects in rats after perinatal exposure to perfluorohexane sulfonate (PFHxS). Sci. Rep. 2020, 10, 2672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassone, C.G.; Vongphachan, V.; Chiu, S.; Williams, K.L.; Letcher, R.J.; Pelletier, E.; Crump, D.; Kennedy, S.W. In ovo effects of perfluorohexane sulfonate and perfluorohexanoate on pipping success, development, mRNA expression, and thyroid hormone levels in chicken embryos. Toxicol. Sci. 2012, 127, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Harris, M.W.; Uraih, L.C.; Birnbaum, L.S. Acute toxicity of perfluorodecanoic acid in C57BL/6 mice differs from 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam. Appl. Toxicol. 1989, 13, 723–736. [Google Scholar] [CrossRef]
- Ren, X.-M.; Qin, W.-P.; Cao, L.-Y.; Zhang, J.; Yang, Y.; Wan, B.; Guo, L.-H. Binding interactions of perfluoroalkyl substances with thyroid hormone transport proteins and potential toxicological implications. Toxicology 2016, 366–367, 32–42. [Google Scholar] [CrossRef]
- Song, M.; Kim, Y.-J.; Park, Y.-K.; Ryu, J.-C. Changes in thyroid peroxidase activity in response to various chemicals. J. Environ. Monit. 2012, 14, 2121–2126. [Google Scholar] [CrossRef]
- Selano, J.; Richardson, V.; Washington, J.; Mazur, C. Characterization of non-radiolabeled Thyroxine (T4) uptake in cryopreserved rat hepatocyte suspensions: Pharmacokinetic implications for PFOA and PFOS chemical exposure. Toxicol. In Vitro 2019, 58, 230–238. [Google Scholar] [CrossRef]
- Xin, Y.; Ren, X.-M.; Ruan, T.; Li, C.-H.; Guo, L.-H.; Jiang, G. Chlorinated Polyfluoroalkylether Sulfonates Exhibit Similar Binding Potency and Activity to Thyroid Hormone Transport Proteins and Nuclear Receptors as Perfluorooctanesulfonate. Environ. Sci. Technol. 2018, 52, 9412–9418. [Google Scholar] [CrossRef]
- Weiss, J.M.; Andersson, P.L.; Lamoree, M.H.; Leonards, P.E.; van Leeuwen, S.P.; Hamers, T. Competitive binding of poly- and perfluorinated compounds to the thyroid hormone transport protein transthyretin. Toxicol. Sci. 2009, 109, 206–216. [Google Scholar] [CrossRef]
- Long, M.; Ghisari, M.; Bonefeld-Jørgensen, E.C. Effects of perfluoroalkyl acids on the function of the thyroid hormone and the aryl hydrocarbon receptor. Environ. Sci. Pollut. Res. Int. 2013, 20, 8045–8056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckalew, A.R.; Wang, J.; Murr, A.S.; Deisenroth, C.; Stewart, W.M.; Stoker, T.E.; Laws, S.C. Evaluation of potential sodium-iodide symporter (NIS) inhibitors using a secondary Fischer rat thyroid follicular cell (FRTL-5) radioactive iodide uptake (RAIU) assay. Arch. Toxicol. 2020, 94, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hallinger, D.R.; Murr, A.S.; Buckalew, A.R.; Lougee, R.R.; Richard, A.M.; Laws, S.C.; Stoker, T.E. High-throughput screening and chemotype-enrichment analysis of ToxCast phase II chemicals evaluated for human sodium-iodide symporter (NIS) inhibition. Environ. Int. 2019, 126, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Kim, Y.-J.; Song, M.-K.; Choi, H.-S.; Park, Y.-K.; Ryu, J.-C. Identification of classifiers for increase or decrease of thyroid peroxidase activity in the FTC-238/hTPO recombinant cell line. Environ. Sci. Technol. 2011, 45, 7906–7914. [Google Scholar] [CrossRef] [PubMed]
- Chambers, W.S.; Hopkins, J.G.; Richards, S.M. A Review of Per- and Polyfluorinated Alkyl Substance Impairment of Reproduction. Front. Toxicol. 2021, 3, 732436. [Google Scholar] [CrossRef] [PubMed]
- Kiess, W.; Häussler, G.; Vogel, M. Endocrine-disrupting chemicals and child health. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101516. [Google Scholar] [CrossRef]
- Bangma, J.; Guillette, T.C.; Bommarito, P.A.; Ng, C.; Reiner, J.L.; Lindstrom, A.B.; Strynar, M.J. Understanding the dynamics of physiological changes, protein expression, and PFAS in wildlife. Environ. Int. 2022, 159, 107037. [Google Scholar] [CrossRef]
- Dankers, A.C.; Roelofs, M.J.; Piersma, A.H.; Sweep, F.C.; Russel, F.G.; van den Berg, M.; van Duursen, M.B.; Masereeuw, R. Endocrine disruptors differentially target ATP-binding cassette transporters in the blood-testis barrier and affect Leydig cell testosterone secretion in vitro. Toxicol. Sci. 2013, 136, 382–391. [Google Scholar] [CrossRef] [Green Version]
- Kummu, M.; Sieppi, E.; Koponen, J.; Laatio, L.; Vähäkangas, K.; Kiviranta, H.; Rautio, A.; Myllynen, P. Organic anion transporter 4 (OAT 4) modifies placental transfer of perfluorinated alkyl acids PFOS and PFOA in human placental ex vivo perfusion system. Placenta 2015, 36, 1185–1191. [Google Scholar] [CrossRef]
- Temkin, A.M.; Hocevar, B.A.; Andrews, D.Q.; Naidenko, O.V.; Kamendulis, L.M. Application of the Key Characteristics of Carcinogens to Per and Polyfluoroalkyl Substances. Int. J. Environ. Res. Public Health 2020, 17, 1668. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinenko, S.A.; Starkova, T.Y.; Kuzmin, A.A.; Tomilin, A.N. Physiological Signaling Functions of Reactive Oxygen Species in Stem Cells: From Flies to Man. Front. Cell Dev. Biol. 2021, 9, 714370. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Marcon, B.H.; Shigunov, P.; Spangenberg, L.; Pereira, I.T.; de Aguiar, A.M.; Amorín, R.; Rebelatto, C.K.; Correa, A.; Dallagiovanna, B. Cell cycle genes are downregulated after adipogenic triggering in human adipose tissue-derived stem cells by regulation of mRNA abundance. Sci. Rep. 2019, 9, 5611. [Google Scholar] [CrossRef] [Green Version]
- Castro, J.P.; Grune, T.; Speckmann, B. The two faces of reactive oxygen species (ROS) in adipocyte function and dysfunction. Biol. Chem. 2016, 397, 709–724. [Google Scholar] [CrossRef] [Green Version]
- de Villiers, D.; Potgieter, M.; Ambele, M.A.; Adam, L.; Durandt, C.; Pepper, M.S. The Role of Reactive Oxygen Species in Adipogenic Differentiation. Adv. Exp. Med. Biol. 2018, 1083, 125–144. [Google Scholar] [CrossRef]
- Zhang, Y.; Khan, D.; Delling, J.; Tobiasch, E. Mechanisms underlying the osteo- and adipo-differentiation of human mesenchymal stem cells. Sci. World J. 2012, 2012, 793823. [Google Scholar] [CrossRef] [Green Version]
- Rinne, N.; Christie, E.L.; Ardasheva, A.; Kwok, C.H.; Demchenko, N.; Low, C.; Tralau-Stewart, C.; Fotopoulou, C.; Cunnea, P. Targeting the PI3K/AKT/mTOR pathway in epithelial ovarian cancer, therapeutic treatment options for platinum-resistant ovarian cancer. Cancer Drug Resist. 2021, 4, 573–595. [Google Scholar] [CrossRef]
- Yudushkin, I. Control of Akt activity and substrate phosphorylation in cells. IUBMB Life 2020, 72, 1115–1125. [Google Scholar] [CrossRef] [Green Version]
- Guru, A.; Issac, P.K.; Velayutham, M.; Saraswathi, N.T.; Arshad, A.; Arockiaraj, J. Molecular mechanism of down-regulating adipogenic transcription factors in 3T3-L1 adipocyte cells by bioactive anti-adipogenic compounds. Mol. Biol. Rep. 2021, 48, 743–761. [Google Scholar] [CrossRef]
- Morrison, D.K. MAP kinase pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirtonia, A.; Sethi, G.; Garg, M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell. Mol. Life Sci. 2020, 77, 4459–4483. [Google Scholar] [CrossRef] [PubMed]
- Kvandová, M.; Majzúnová, M.; Dovinová, I. The role of PPARgamma in cardiovascular diseases. Physiol. Res. 2016, 65 (Suppl. 3), S343–S363. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Kim, S.; Chung, H.-T.; Pae, H.-O. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013, 528, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Watkins, A.M.; Wood, C.R.; Lin, M.T.; Abbott, B.D. The effects of perfluorinated chemicals on adipocyte differentiation in vitro. Mol. Cell. Endocrinol. 2015, 400, 90–101. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, R.; Laviola, L.; Giorgino, F.; Unfer, V.; Bettocchi, S.; Scioscia, M. PKB/Akt and MAPK/ERK phosphorylation is highly induced by inositols: Novel potential insights in endothelial dysfunction in preeclampsia. Pregnancy Hypertens. 2017, 10, 107–112. [Google Scholar] [CrossRef]
- Shao, J.; Yamashita, H.; Qiao, L.; Friedman, J.E. Decreased Akt kinase activity and insulin resistance in C57BL/KsJ-Leprdb/db mice. J. Endocrinol. 2000, 167, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Shabalina, I.G.; Kramarova, T.V.; Mattsson, C.L.; Petrovic, N.; Rahman Qazi, M.; Csikasz, R.I.; Chang, S.C.; Butenhoff, J.; DePierre, J.W.; Cannon, B.; et al. The Environmental Pollutants Perfluorooctane Sulfonate and Perfluorooctanoic Acid Upregulate Uncoupling Protein 1 (UCP1) in Brown-Fat Mitochondria through a UCP1-Dependent Reduction in Food Intake. Toxicol. Sci. 2015, 146, 334–343. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Xie, G.; Sun, X.; Li, Q.; Zhong, W.; Qiao, P.; Sun, X.; Jia, W.; Zhou, Z. High fat diet feeding exaggerates perfluorooctanoic acid-induced liver injury in mice via modulating multiple metabolic pathways. PLoS ONE 2013, 8, e61409. [Google Scholar] [CrossRef] [Green Version]
- Lind, P.M.; Lind, L.; Salihovic, S.; Ahlström, H.; Michaelsson, K.; Kullberg, J.; Strand, R. Serum levels of perfluoroalkyl substances (PFAS) and body composition—A cross-sectional study in a middle-aged population. Environ. Res. 2022, 209, 112677. [Google Scholar] [CrossRef]
- Starling, A.P.; Adgate, J.L.; Hamman, R.F.; Kechris, K.; Calafat, A.M.; Ye, X.; Dabelea, D. Perfluoroalkyl Substances during Pregnancy and Offspring Weight and Adiposity at Birth: Examining Mediation by Maternal Fasting Glucose in the Healthy Start Study. Environ. Health Perspect. 2017, 125, 067016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.-H.; Ng, S.; Hsieh, C.-J.; Lin, C.-C.; Hsieh, W.-S.; Chen, P.-C. The impact of prenatal perfluoroalkyl substances exposure on neonatal and child growth. Sci.Total Environ. 2017, 607–608, 669–675, Erratum in Sci. Total Environ. 2018, 644, 1653. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, T.; Nishimura, T.; Nomura, Y.; Iwabuchi, T.; Itoh, H.; Takizawa, T.; Tsuchiya, K.J. Umbilical cord serum concentrations of perfluorooctane sulfonate, perfluorooctanoic acid, and the body mass index changes from birth to 5 1/2 years of age. Sci. Rep. 2021, 11, 19789. [Google Scholar] [CrossRef] [PubMed]
- Tanner, E.M.; Bornehag, C.G.; Gennings, C. Dynamic growth metrics for examining prenatal exposure impacts on child growth trajectories: Application to perfluorooctanoic acid (PFOA) and postnatal weight gain. Environ. Res. 2020, 182, 109044. [Google Scholar] [CrossRef] [PubMed]
- Starling, A.P.; Liu, C.; Shen, G.; Yang, I.V.; Kechris, K.; Borengasser, S.J.; Boyle, K.E.; Zhang, W.; Smith, H.A.; Calafat, A.M.; et al. Prenatal Exposure to Per- and Polyfluoroalkyl Substances, Umbilical Cord Blood DNA Methylation, and Cardio-Metabolic Indicators in Newborns: The Healthy Start Study. Environ. Health Perspect. 2020, 128, 127014. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef] [PubMed]
- White, S.S.; Calafat, A.M.; Kuklenyik, Z.; Villanueva, L.; Zehr, R.D.; Helfant, L.; Strynar, M.J.; Lindstrom, A.B.; Thibodeaux, J.R.; Wood, C.; et al. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol. Sci. 2007, 96, 133–144. [Google Scholar] [CrossRef]
- Lau, C.; Thibodeaux, J.R.; Hanson, R.G.; Narotsky, M.G.; Rogers, J.M.; Lindstrom, A.B.; Strynar, M.J. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol. Sci. 2006, 90, 510–518. [Google Scholar] [CrossRef] [Green Version]
- Koustas, E.; Lam, J.; Sutton, P.; Johnson, P.I.; Atchley, D.S.; Sen, S.; Robinson, K.A.; Axelrad, D.A.; Woodruff, T.J. The Navigation Guide—Evidence-based medicine meets environmental health: Systematic review of nonhuman evidence for PFOA effects on fetal growth. Environ. Health Perspect. 2014, 122, 1015–1027. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, X.; Chen, L.; Qin, Y.; Xu, Y.; Tian, Y.; Chen, L. Exposure of female mice to perfluorooctanoic acid suppresses hypothalamic kisspeptin-reproductive endocrine system through enhanced hepatic fibroblast growth factor 21 synthesis, leading to ovulation failure and prolonged dioestrus. J. Neuroendocrinol. 2020, 32, e12848. [Google Scholar] [CrossRef]
- Wang, X.; Bai, Y.; Tang, C.; Cao, X.; Chang, F.; Chen, L. Impact of Perfluorooctane Sulfonate on Reproductive Ability of Female Mice through Suppression of Estrogen Receptor α-Activated Kisspeptin Neurons. Toxicol. Sci. 2018, 165, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard-Olesen, C.; Ghisari, M.; Bonefeld-Jørgensen, E.C. Activation of the estrogen receptor by human serum extracts containing mixtures of perfluorinated alkyl acids from pregnant women. Environ. Res. 2016, 151, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, Q.; Chen, M.; Yang, J.; Wang, R.; Zhong, W.; Zhu, L.; Yang, L. Antagonistic Estrogenic Effects Displayed by Bisphenol AF and Perfluorooctanoic Acid on Zebrafish (Danio rerio) at an Early Developmental Stage. Environ. Sci. Technol. Lett. 2018, 5, 655–661. [Google Scholar] [CrossRef]
- Mora, A.M.; Oken, E.; Rifas-Shiman, S.L.; Webster, T.F.; Gillman, M.W.; Calafat, A.M.; Ye, X.; Sagiv, S.K. Prenatal Exposure to Perfluoroalkyl Substances and Adiposity in Early and Mid-Childhood. Environ. Health Perspect. 2017, 125, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Halldorsson, T.I.; Rytter, D.; Haug, L.S.; Bech, B.H.; Danielsen, I.; Becher, G.; Henriksen, T.B.; Olsen, S.F. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: A prospective cohort study. Environ. Health Perspect. 2012, 120, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Contempré, B.; Jauniaux, E.; Calvo, R.; Jurkovic, D.; Campbell, S.; de Escobar, G.M. Detection of thyroid hormones in human embryonic cavities during the first trimester of pregnancy. J. Clin. Endocrinol. Metab. 1993, 77, 1719–1722. [Google Scholar] [CrossRef] [Green Version]
- Vulsma, T.; Gons, M.H.; de Vijlder, J.J. Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N. Engl. J. Med. 1989, 321, 13–16. [Google Scholar] [CrossRef]
- Fisher, D.A. Fetal thyroid function: Diagnosis and management of fetal thyroid disorders. Clin. Obstet. Gynecol. 1997, 40, 16–31. [Google Scholar] [CrossRef]
- Wang, Y.; Rogan, W.J.; Chen, P.-C.; Lien, G.-W.; Chen, H.-Y.; Tseng, Y.-C.; Longnecker, M.P.; Wang, S.-L. Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan maternal and infant cohort study. Environ. Health Perspect. 2014, 122, 529–534. [Google Scholar] [CrossRef] [Green Version]
- Idris, I.; Srinivasan, R.; Simm, A.; Page, R.C. Maternal hypothyroidism in early and late gestation: Effects on neonatal and obstetric outcome. Clin. Endocrinol. 2005, 63, 560–565. [Google Scholar] [CrossRef]
- Sahu, M.T.; Das, V.; Mittal, S.; Agarwal, A.; Sahu, M. Overt and subclinical thyroid dysfunction among Indian pregnant women and its effect on maternal and fetal outcome. Arch. Gynecol. Obstet. 2010, 281, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Boesen, S.A.H.; Long, M.; Wielsøe, M.; Mustieles, V.; Fernandez, M.F.; Bonefeld-Jørgensen, E.C. Exposure to Perflouroalkyl acids and foetal and maternal thyroid status: A review. Environ. Health 2020, 19, 107. [Google Scholar] [CrossRef] [PubMed]
- Vernier, M.; Dufour, C.R.; McGuirk, S.; Scholtes, C.; Li, X.; Bourmeau, G.; Kuasne, H.; Park, M.; St.-Pierre, J.; Audet-Walsh, E.; et al. Estrogen-related receptors are targetable ROS sensors. Genes Dev. 2020, 34, 544–559. [Google Scholar] [CrossRef] [PubMed]
- Vuguin, P.M. Animal models for small for gestational age and fetal programming of adult disease. Horm. Res. Paediatr. 2007, 68, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Halappanavar, S.; van den Brule, S.; Nymark, P.; Gaté, L.; Seidel, C.; Valentino, S.; Zhernovkov, V.; Høgh Danielsen, P.; De Vizcaya, A.; Wolff, H.; et al. Adverse outcome pathways as a tool for the design of testing strategies to support the safety assessment of emerging advanced materials at the nanoscale. Part. Fibre Toxicol. 2020, 17, 16. [Google Scholar] [CrossRef]
- Schaefer, M.H.; Yang, J.-S.; Serrano, L.; Kiel, C. Protein conservation and variation suggest mechanisms of cell type-specific modulation of signaling pathways. PLoS Comput. Biol. 2014, 10, e1003659. [Google Scholar] [CrossRef]
- Peiris, T.H.; Ramirez, D.; Barghouth, P.G.; Oviedo, N.J. The Akt signaling pathway is required for tissue maintenance and regeneration in planarians. BMC Dev. Biol. 2016, 16, 7. [Google Scholar] [CrossRef]


| Source | Type of Analysis | No of Studies (Publication Date) | Analyte(s) | Surveyed Birth Outcome | Main Results | Conclusions |
|---|---|---|---|---|---|---|
| Bach et al., 2015 [28] | Systematic review | 14 (2004–2013) | PFOA, PFOS | Birth weight Low birth weight a Small for gestational age b Birth weight z-scores c | PFOA exposure associated with decreased measures of continuous birth weight in all studies at different magnitudes, with many results being statistically insignificant PFOS: no clear trend for effects on birth weight | “The impact on public health is unclear” |
| Negri et al., 2017 [29] | Systematic review | 16 (up to 2015) | PFOA, PFOS | Birth weight | PFOA: −12.8 g/ng/mL (−27.1 g per increase of 1 loge ng/mL) PFOS: −0.92 g/ng/mL (−46.1 g per increase of 1 loge ng/mL) | “…no quantitative toxicological evidence to support the epidemiological association, thus reducing the biological plausibility of a causal relationship” |
| Govarts et al., 2018 [6] | Pooled analysis | 7 birth cohorts 5446 mother–child pairs | PFOA, PFOS | Small for gestational age b | PFOA: Higher levels associated with greater risk of SGA (OR: 1.64) PFOS: Higher levels associated with greater risk of SGA (OR: 1.63) in newborns of mothers who smoked during pregnancy (but decreased risk in newborns of non-smoking mothers (OR: 0.66)) | “Prenatal environmental exposure to perfluorinated compounds with endocrine disrupting properties may contribute to the prevalence of SGA. We found indication of effect modification by child’s sex and smoking during pregnancy. The direction of the associations differed by chemical and these effect modifiers, suggesting diverse mechanisms of action and biological pathways” |
| Dzierlenga et al., 2020 [30] | Random-effects meta-regression | 29 (up to 2019) | PFOS | Birth weight | −3.22 g/ng/mL (all) −1.35 g/ng/mL (early group d) −7.17 g/ng/mL (later group d) | “...when blood was drawn at the very beginning of pregnancy, there was essentially no relation of birth weight to PFOS”, “stronger inverse association in Asian studies”, “The evidence was weakly or not supportive of a causal association” |
| Cao et al., 2021 preprint [31] | Meta-analysis (fixed-effect and random-effect models) | 6 (2009–2017) | PFOA, PFOS | Low birth weight a | PFOA: OR = 0.90 PFOS: OR = 1.32 (America: OR = 1.44) | “...study provided a systematic review and meta-analysis evidence for the relationship between maternal PFASs exposure and LBW of offspring through a small number of studies. Researchers should conduct further studies between different regions” |
| Lee et al., 2021 [32] | Systematic review | 90 (2007–2021) | PFOA, PFOS, 11 other PFAS | Birth weight Birth length Ponderal Index e Gestational age | Most studies suggest that prenatal PFAS exposure (especially long-chain PFAS) may affect fetal growth | “The current epidemiologic evidence has mostly suggested that prenatal PFAS exposures may impair fetal growth... The mechanisms through which PFAS affect early-life physical development in humans remain unclear” |
| MeSH Terms | Other Search Terms |
|---|---|
| Infant, Small for Gestational Age | Amino acids |
| Infant, Small for Gestational Age/growth and development | Nutrients |
| Infant, Small for Gestational Age/blood | Glucose |
| Infant, Small for Gestational Age/metabolism | Fatty acids |
| Infant, Small for Gestational Age/physiology | Fetal growth restriction |
| Premature Birth | Intrauterine growth retardation |
| Pre-Eclampsia | Intrauterine growth restriction |
| Receptor, Fibroblast Growth Factor, Type 1 | Placenta malperfusion |
| Placenta Diseases | Vascular endothelial growth factor |
| Placenta Growth Factor | Flt-1 |
| Placenta Growth Factor, PLGF-1 Isoform | Thiol adduct |
| Receptors, Vascular Endothelial Growth Factor | Thio/seleno-protein |
| Receptors, Androgen | Oxidative stress |
| Receptors, Estrogen | DNA polymerase gamma |
| Small for Gestational Age | |
| Small for Gestational Age/growth and development | |
| Small for Gestational Age/blood | |
| Small for Gestational Age/metabolism | |
| Small for Gestational Age/physiology | |
| Fetal growth restriction | |
| Intrauterine growth retardation | |
| Intrauterine growth restriction | |
| IUGR | |
| Inhibition Cytochrome P450 enzyme activity | |
| Inhibition CYP17A1 activity | |
| Decreased Aromatase mRNA | |
| Decreased Cyp19a1 mRNA | |
| Fetal growth | |
| AO | Increase, Growth inhibition |
| AO | Growth, reduction |
| AO | Decrease, Growth |
| MIE | Inhibition, VegfR2 |
| KE | Decreased, angiogenesis |
| KE | Defect of Embryogenesis |
| KE | Decrease, Growth |
| KE | Reduction, Progesterone synthesis |
| Oxidative stress | |
| MIE | Activation, NRF2 |
| KE | ROS formation |
| KE | Increase, Oxidative Stress |
| KE | Activation, PMK-1 P38 MAPK |
| KE | Down Regulation, GSS and GSTs gene |
| KE | Glutathione synthesis |
| KE | Glutathione homeostasis |
| MIE | Thiol group of chemicals interact with sulfhydryl groups of proteins to form thiol adducts |
| MIE | Inhibition of mitochondrial DNA polymerase gamma |
| KE | Dysfunction, Mitochondria |
| MIE | Binding, Thiol/seleno-proteins involved in protection against oxidative stress |
| Signaling pathways | |
| KE | Activation, AKT2 |
| KE | Activation, HIF-1 |
| KE | Activation, JAK/STAT pathway |
| KE | Activation, TGF-beta pathway |
| KE | Activation, JNK |
| MIE | Wnt ligand stimulation |
| KE | Inhibition, Wnt pathway |
| KE | Frizzled activation |
| KE | Alteration, Wnt pathway |
| Endocrine related pathways | |
| MIE | Activation, Androgen receptor |
| MIE | Decreased, Androgen receptor activity |
| MIE | Activation, Estrogen receptor |
| KE | Increased, Estrogen receptor activity |
| KE | Increased, ER activity |
| KE | Decrease, testosterone synthesis |
| KE | Decrease, testosterone level |
| KE | Decrease, dihydrotestosterone level |
| KE | Decrease, DHT level |
| KE | Decrease, androgen receptors (AR) activation |
| KE | Decrease, AR activation |
| KE | Reduction, 17-OH-pregnenolone conversion in DHEA |
| KE | Reduction, 17-OH-progesterone conversion in androstenedione |
| KE | Thyroid hormone disruption |
| Others | |
| MIE | Inhibition, Cytochrome P450 enzyme (CYP17A1) activity |
| MIE | Binding of substrate, endocytic receptor |
| MIE | Inhibition, Aromatase |
| KE | Decreased, Aromatase (Cyp19a1) mRNA |
| KE | Perturbation of cholesterol |
| KE | GSK3beta inactivation |
| KE | β-catenin activation |
| PFAS, General Terms | |
|---|---|
| PFAS, Perfluoroalkyl substances | |
| Perfluoroalkyl substances | |
| Perfluoroalkyl acids | |
| Perfluoroalkyl carboxylates (PFCAs) | |
| Perfluroalkylated substances | |
| PFC, Perfluorinated compound | |
| Perfluorinated sulfonates (PFSAs) | |
| PFAS compounds * | |
| PFCAs | |
| C8 | PFOA, perfluorooctanoic acid |
| C9 | PFNA, perfluorononanoic acid |
| C10 | PFDA, perfluorodecanoic acid or perfluoro-n-decanoic acid |
| PFSAs | |
| C6 | PFHxS, perfluorohexanesulfonic acid or perfluoro-1-hexanesulfonate |
| C8 | PFOS, perfluorooctane sulfonic acid or perfluorooctane sulfonate |
| Publication Information | Study Setup | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Species | Solvent | Body Weight | Fetal Weight | Offspring Weight | Rep/Dev | Amino acids | Glucose | Lipids | Liver | Oxidative Stress | Lethality | Specific Genes | Other AO |
| PFOS | ||||||||||||||
| Kim et al., 2020 [53] | C. elegans | ↓ | ↑↓ | ↑↓ | ↑ | ↑ | ||||||||
| Kim et al., 2021 [54] | Drosophila | Aceton | ↓ | ↓ | ↑↓ | ↑ | ||||||||
| Lee et al., 2015 [55] | Female CD-1 mice | DMSO | ↑↓ | ↑ | ||||||||||
| Li et al., 2020 [56] | Adult CD-1 mice | DMSO | DNA Methylation | |||||||||||
| Li et al., 2020 [57] | C. elegans | Water | ↑↓ | |||||||||||
| Ortiz-Villanueva et al., 2018 [58] | Zebrafish | DMSO | ↑↓ | Metabolome | ||||||||||
| Park et al., 2020 [59] | Macrophthalmus japonicus crab | Involvement of MAPK/p38 | ||||||||||||
| Qiu et al., 2016 [60] | Male ICR mice | DMSO | – | ↑ | ||||||||||
| Seyoum et al., 2020 [61] | Daphnia | Water | ↓ | ↓ | ↑↓ | ↑ | ||||||||
| Wan et al., 2020 [62] | CD1 mice | DMSO | ↓ | ↓ | SNAT4 | |||||||||
| Wang et al., 2020 [63] | Dugesia japonica | DMSO | ↑ | SOD, CAT, GPx1 | ||||||||||
| Xia et al., 2018 [64] | Anodonta woodiana | DMSO | ↑ | |||||||||||
| Yue et al., 2020 [65] | C. elegans | Water | ↓ | ↓ | ↑↓ | ↑ | Metabolism | |||||||
| Zhang et al., 2020 [66] | Manila clam | DMSO | ↑↓ | Metabolism/Genes | ||||||||||
| PFOA | ||||||||||||||
| Du et al., 2018 [67] | Male Balb/c mice | DMSO | ↓ | ↑ | ↑ | ↑ | ||||||||
| Guruge et al., 2006 [68] | Male Sprague–Dawley rats | ↑↓ | ↑↓ | ↑↓ | Transcriptome | Gene expression | ||||||||
| Kim et al., 2020 [53] | C. elegans | ↓ | ↑↓ | ↑↓ | ↑ | ↑ | Metabolism; Lipidomic | |||||||
| Li et al., 2019 [69] | Kunming mice | ↓ | ↑↓ | ↑ | ↑ | Gene expression | ||||||||
| Li et al., 2021 [70] | M. edulis | Water | ↑ | CAT/SOD/GPx | ||||||||||
| Liu et al., 2015 [71] | Male mice | Water | ↓ | ↑ | ||||||||||
| Liu et al., 1996 [72] | Male rats | unclear | ↓ | ↑ | ||||||||||
| Salimi et al., 2019 [73] | Mouse | ↓ | ↑ | |||||||||||
| Seyoum et al., 2020 [61] | Daphnia | – | ↓ | ↑↓ | ↑ | |||||||||
| Wang et al., 2010 [74] | Drosophila | ↓ | ↓ | ↓ | Reduced longevity of males | |||||||||
| Xia et al., 2018 [64] | Anodonta woodiana | DMSO | ↑ | |||||||||||
| Yan et al., 2015 [75] | Male Balb/c mice | Water | ↑↓ | ↑↓ | ↑↓ | Akt, GSK | ||||||||
| Yang 2010 [76] | Oryzias latipes | Water | – | PPR alpha | ||||||||||
| Publication Information | Study Setup | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Cell System | Species | Reproduction | Amino Acids | Glucose | Lipids/Fats | Oxidative Stress | Cytotoxic/Reduced Cell Number | Spec. Genes | Other AO |
| PFOS | ||||||||||
| Chiu et al., 2018 [77] | Not described | Tox-Screening; ToxCast; Tox21 | ↑ | Many different AO | ||||||
| Gorrochategui et al., 2014 [78] | JEG-3 | Human | ↑ | |||||||
| Gorrochategui et al., 2016 [79] | A6 Kidney Epithelial Cells | Xenopus laevis | ↑ | |||||||
| Li et al., 2020 [56] | HTR-8/SVneo | Human | ↑ | |||||||
| Reistad et al., 2013 [80] | Cerebellar granule cells | Rat | ↑ | ↑ | ||||||
| Sun et al., 2018 [81] | SH-SY5Y Cell | Human | ↑ | ↑ | NRF2, HO-1 | |||||
| Sun et al., 2019 [82] | SH-SY5Y Cell | Human | ↑ | ↑ | JNK-1 | |||||
| Tang et al., 2017 [83] | ES cell line D3 | Mouse | ↑ | ↑ | ↑ | Mfn1, Mfn2, mTOR, RICTOR | Ca2+ flux is impaired | |||
| Wang et al., 2015 [84] | HAPI microglial cells | Rat | ↑ | ↑ | ERK, JNK, p38 | |||||
| Wei et al., 2009 [85] | Primary hepatocytes | Gobiocypris rarus (fish) | ↑ | ↑ | ↑ | Gene expression | ||||
| Xu et al., 2016 [86] | 3T3-L1 pre-adipocytes | Mouse | ↑ | ↑↓ | ↑ | NRF2, Lpl, NQo1, PPAR, FABP4 | ||||
| Zarei et al., 2018 [87] | Lymphocytes | Human | ↑ | ↑ | ||||||
| PFOA | ||||||||||
| Chiu et al., 2018 [77] | Not described | Tox-Screening; ToxCast; Tox21 | ↑ | Many different AOs | ||||||
| Gorrochategui et al., 2014 [78] | JEG-3 | Human | ↑ | |||||||
| Gorrochategui et al., 2016 [79] | A6 Kidney Epithelial Cells | Xenopus laevis | ↑ | |||||||
| Lu et al., 2016 [88] | Sperm cells | Mouse | ↓ | ↓ | ↑ | ↑ | FABP3/4/6/KAR/ELOVL5 | AKT | ||
| Mashayekhi et al., 2015 [89] | Rat mitochondria (liver/brain) | Rat | ↑ | – | No changes in GSH levels | |||||
| Reistad et al., 2013 [80] | Cerebellar granule cells | Rat | ↑ | |||||||
| Suh et al., 2017 [90] | RIN-m5F cells | Rat | ↑ | ↑ | ||||||
| Tang et al., 2018 [91] | Primary lymphocytes | C. auratus | ↑ | ↑ | ||||||
| Tian et al., 2021 [92] | RAW264.7 | Mouse | ↑↓ | ↑↓ | ↑ | ↑ | ||||
| Wei et al., 2009 [85] | Primary hepatocytes | Gobiocypris rarus (fish) | ↑ | ↑ | – | |||||
| PFNA | ||||||||||
| Gorrochategui et al., 2014 [78] | JEG-3 | Human | ↑ | |||||||
| Wei et al., 2009 [85] | Primary hepatocytes | Gobiocypris rarus (fish) | ↑ | ↑ | – | |||||
| PFHxS | ||||||||||
| Gorrochategui et al., 2014 [78] | JEG-3 | Human | – | |||||||
| Lee et al., 2014 [93] | Neuronal cells | Rat | ↑ | ↑ | ||||||
| Lee et al., 2014 [94] | PC12 | Rat | ↑ | ↑ | ||||||
| PFDA | ||||||||||
| Dong et al., 2017 [95] | AGS gastric epithelial cells | Human | ↓ | |||||||
| Kleszczyński et al., 2011 [96] | HCT116 | Human | Ca ions inside mitochondria | |||||||
| Wei et al., 2009 [85] | Primary hepatocytes | Gobiocypris rarus (fish) | ↑ | – | ||||||
| Xu et al., 2019 [97] | Hepatic cells | Mouse | ↑ | DNA Damage | ||||||
| Mixture | ||||||||||
| Wei et al., 2009 [85] | Primary hepatocytes | Gobiocypris rarus (fish) | ↑ | – | ||||||
| Publication Information | Study Setup | Cellular Signaling | |||||
|---|---|---|---|---|---|---|---|
| Authors | Species | Target Tissue | P-AKT (Thr308) | p-AKT (S473) | P38 mRNA | PPARα mRNA | PPARγ mRNA |
| PFOS | |||||||
| Park et al., 2020 [59] | Crab (Macrophthalmus japonicus) | Gill, hepatopancreas | ↑ | ||||
| Qiu et al., 2016 [60] | Mouse (male ICR mice 8 weeks of age) | Testes | ↑ | ||||
| Xu et al., 2016 [86] | Mouse (C57BL/6 mice 10 weeks of age) | Epididymal white adipose tissue | ↑ | ||||
| Zhang and Sun et al., 2020 [66] | Clam (R. philippinarum) | Hepatopancreas | ↑ | ||||
| PFOA | |||||||
| Du et al., 2018 [67] | Mouse (male Balb/c mice 6–7 weeks of age) | Adipose tissue | ↓ | ||||
| Lu et al., 2016 [88] | Mouse (male Balb/c mice 6–8 weeks of age) | Epidymis | ↑ | ||||
| Yan et al., 2015 [75] | Mouse (male Balb/c mice 6–8 weeks of age) | Liver | ↑ | ↑ | |||
| Yan et al., 2015 [75] | Mouse (male Balb/c mice 6–8 weeks of age) | Muscle | ↑ | ||||
| Yan et al. 2015 [75] | Mouse (male Balb/c mice 6–8 weeks of age) | White adipose tissue | ↓ | ||||
| Yang 2010 [98] | Fish (male medaka fish) | Liver | ↑ | ||||
| Publication Information | Study Setup | Cellular Signaling | ||||
|---|---|---|---|---|---|---|
| Authors | Species | Cell Type | P-ERK | P-JNK | P-p38 | PPARγ mRNA |
| PFOS | ||||||
| Qiu et al., 2016 [60] | Mouse (male ICR mice 8 weeks of age) | Primary Sertoli cells | ↑ | |||
| Sun et al., 2019 [82] | Human | SH-SY5Y (neuroblastoma) | ↑ | |||
| Wang et al., 2015 [84] | Rat | HAPI (microglia-like cell line) | ↑ | ↑ | – | |
| Xu et al., 2016 [86] | Mouse | Adipocytes derived from 3T3-L1 preadipocyte cell line | ↑ | |||
| PFHxS | ||||||
| Lee et al., 2014 [94] | Rat | PC12 (adrenal gland) | ↑ | ↑ | ↑ | |
| Lee et al., 2014 [93] | Rat (7-day old Sprague–Dawley rat pups) | Primary cerebella granular cells | ↑ | ↑ | ↑ | |
| Publication information | Study Setup | Estrogenic and Androgenic Related Results | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Species | Estrogen levels | ER Transcription (mRNA) | ER Expression (Protein) | VTG | Testosterone Levels | AR Transcription (mRNA) | AR Expression(protein) | CYP | Other Directly Estrogenic/Androgenic Related Effects |
| PFOS | ||||||||||
| Bao et al., 2019 [102] | Female zebrafish | ↑↓ | ↑↓ | ↑↓ | Altered gene expression along the HPGL axis | |||||
| Bao et al., 2020 [103] | Male zebrafish | ↑ | ↑ | ↓ | ↓FSH and LH receptor in gonads, ↓ expression of GnRH, GNRHr, FSH, and LH in brain, impaired sexual behavior | |||||
| Benninghoff et al., 2011 [104] | Juvenile rainbow trout | – | ||||||||
| Biegel et al., 1995 [105] | Rats (male CD) | ↑ | ↑ | ↑CYP19 | ||||||
| Chen et al., 2016 [106] | Zebra fish (Post-fertilization) | ↑ | ↑ | ↓ | CYP19A (↑female) / (↓male) | ↑ amh (gonad), structural changes in gonads | ||||
| Du et al., 2013 [107] | Zebrafish embryo | ↑↓ | ↓CYP17, CYP19a, CYP19b | |||||||
| Qu et al., 2016 [108] | Mouse (C57 male) | – | ↑↓ | ↓ | ↓ sperm concentration, vacuolations observed in spermatogonia, spermatocytes and Leydig cells, ↑ incidence of apoptotic cells (testes) | |||||
| Qiu et al., 2020 [109] | Famale Spague Dawley rat | ↑ | ↑ | |||||||
| Qiu et al., 2021 [110] | Mouse (ICR male) | – | ↓ | No effect on LH or FSH, ↓ sperm count, damaged testicular interstitium morphology | ||||||
| Rodríguez-Jorquera et al., 2019 [111] | Fathead minnow (Pimephales promelas) | ↑ | ||||||||
| Rosen et al., 2017 [112] | Mouse (wt and ppara-null) | Gene-expression: ↓ male-specific genes, ↑ female-specific genes | ||||||||
| Xin et al., 2020 [113] | Zebra fish | ↑ | ↑ | ↑ | ↑CYP19a, ↓ CYP19b | Altered spermgenesis | ||||
| Xu et al., 2017 [114] | Mouse (-/- and +/+ ERβ) | ↑ | Only in ERβ +/+ mice: hydropic degeneration and vacuolation in hepatocytes, increase cholesterol and bile acid, altered liver genes. | |||||||
| Zhang and Lu et al., 2020 [115] | Rats (pregnant Sprague-Dawley) | ↓ | ↓CYP11A1, CYP17A1, Hsd17b3 | ↓ Dhh and SOX9 (sertoli cells), affected proliferation (leydig stem cells) | ||||||
| Zhao et al., 2014 [116] | Rats (pregnant Sprague-Dawley) | ↓ | ↓Cyp11a1 Cyp17a, Hsd3b1 | ↓ AGD and testicular weights (male pups), impaired fetal Leydig cells, ↓ fetal Leydig cells number | ||||||
| Zhong et al., 2016 [117] | Mouse (C57BL/6) | ↑ | ↓ | |||||||
| PFOA | ||||||||||
| Benninghoff et al., 2011 [104] | Juvenile rainbow trout | ↑ | ||||||||
| Lu et al., 2019 [118] | Rat (Sprague-Dawley with eliminated Leydig cells) | ↓ | ↓CYP11A1, CYP17A1 | No effect on serum FSH and LH, ↓ expression of Lhcgr, Scarb1, Star, Hsd3b1 and Hsd11b1 in leydig cells, affected proliferation of stem Leydig cells | ||||||
| Qiu et al., 2020 [109] | Female Sprague Dawley rat | ↑ | ↑ | |||||||
| Rosen et al., 2017 [112] | Mouse (wt and ppara-null) | ↓ expression of male-specific genes, ↑ expression of female-specific genes | ||||||||
| Wei et al., 2007 [119] | Freshwater rare minnow | ↑ | ↑ | ↓ Degenerating vitellogenic-stage oocytes | ||||||
| Xin et al., 2019 [120] | Zebra fish | ↑ | ↑ | |||||||
| Yao et al., 2014 [121] | Female CD-1 mouse | No effect of ER target genes | ||||||||
| Zhao et al., 2010 [122] | Female C57Bl/6 mice | – | ↑ | ↑ serum progesterone, ↑ mammary gland responses to estrogen and progesterone, ↑ liver steroid hormone metabolic enzyme gene expressions, no effect on SHBG | ||||||
| PFNA | ||||||||||
| Benninghoff et al., 2011 [104] | Juvenile rainbow trout | ↑ | ||||||||
| Feng et al., 2009 [123] | Rat (Sprague–Dawley male) | ↑ | ↑↓ | No effect on FSH and LH | ||||||
| Rosen et al., 2017 [112] | Mouse (wt and ppara-null) | ↓ expression of male-specific genes, ↑ expression of female-specific genes | ||||||||
| Singh et al., 2019 [124] | Mouse (prepubertal Parkers male) | ↓CYP11A | ||||||||
| Singh et al., 2019 [125] | Mouse (prepubertal Parkers male) | ↓ | ↓ | ↓ Impairment in testicular functions, Decreased overall germ cell transformation | ||||||
| PFHxS | ||||||||||
| Rosen et al., 2017 [112] | Mouse (wt and ppara-null) | ↓ expression of male-specific genes, ↑ expression of female-specific genes | ||||||||
| PFDA | ||||||||||
| Benninghoff et al., 2011 [104] | Juvenile rainbow trout | ↑ | ||||||||
| Mixture | ||||||||||
| Benninghoff et al., 2011 [104] | Juvenile rainbow trout | ↑ | ||||||||
| Rodríguez-Jorquera et al., 2019 [111] | Fathead minnow (Pimephales promelas) | ↑ | ||||||||
| Authors | Species | Estrogen activity | ERα Expression | ERβ Expression | E2 Secretion/Production | Androgen activity | AR Protein | T Secretion/Production | CYP Enzyme Activities | Other Estrogen-Related Effects | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| reporter gene | E-screen | ||||||||||
| PFOS | |||||||||||
| Xin et al., 2020 [113] | Human | ↑ | ↑ | ||||||||
| Gogola et al., 2020 [126] | Human | ↓ | ↑2-OHE1/E2 ratio | ||||||||
| Human | ↑ | ↓ 2-OHE1, 16-OHE1, 2OHE1/E2 ratio, 16-OHE1/E2 ratio | |||||||||
| Halsne et al., 2016 [127] | Human | Normal acini maturation affected, ER-independent mechanisms to normal development of glandular breast tissue | |||||||||
| Xu et al., 2017 [114] | Human | ↑ | |||||||||
| Benninghoff et al., 2011 [104] | Human | ↑ | |||||||||
| Maras et al., 2006 [128] | Human | – | Altered expression of estrogen-responsive biomarker genes | ||||||||
| Li et al., 2020 [129] | Human | ↑ | Altered expression of estrogen-responsive biomarker genes | ||||||||
| Ishibashi et al., 2008 [130] | Yeast | – | |||||||||
| Behr et al., 2018 [101] | Human | ↑ | – | – | Increased progesterone and estrone, no effect on estrogen- or androgen-responsive genes, | ||||||
| Du et al., 2013 [107] | Monkey and human | ↑ | ↑ | – | ↓ | Altered gene expression | |||||
| Rosen et al., 2017 [112] | Human | ↑ | – | ||||||||
| Biegel et al., 1995 [105] | Rat | – | ↓ | ||||||||
| Kjeldsen et al., 2013 [99] | Human and hamster | ↑ | ↓ | Aromatase unchanged | |||||||
| Kang et al., 2016 [100] | Human | ↓ | ↑ | – | ↓ | ↑CYP17, 3b-hsd2, cyp19 | ↑ Estrone | ||||
| PFOA | |||||||||||
| Xin et al., 2019 [120] | Human | ↑ | |||||||||
| Yao et al., 2014 [121] | Human | – | |||||||||
| Gogola et al., 2020 [126] | Human | ↓ | ↑2-OHE1/E2 ratio | ||||||||
| Human | ↑ | ↓2-OHE1, 16-OHE1, 2OHE1/E2 ratio, 16-OHE1/E2 ratio | |||||||||
| Halsne et al., 2016 [127] | Human | Normal acini maturation not affected | |||||||||
| Benninghoff et al., 2011 [104] | Human | ↑ | |||||||||
| Maras et al., 2006 [128] | Human | – | Altered expression of estrogen-responsive biomarker genes | ||||||||
| Li et al., 2020 [129] | Human | ↑ | Altered expression of estrogen-responsive biomarker genes | ||||||||
| Ishibashi et al., 2008 [130] | Yeast | – | |||||||||
| Behr et al., 2018 [101] | Human | ↑ | – | – | ↑CYP21A2 | ↑ Estrone, no effect on estrogen- or androgen-responsive genes | |||||
| Buhrke et al., 2015 [131] | Human | ↑↓ | |||||||||
| Rosen et al., 2017 [112] | Human | ↑ | – | ||||||||
| Rosenmai et al., 2013 [132] | Human and hamster | ↑↓ | – | – | Unchanged CYP11A, CYP17 or CYP21 | ↑Estrone, ↓ androstenedione, no effect on production of progesterone, 17-OH progesterone, or DHEA | |||||
| Kjeldsen et al., 2013 [99] | Human and hamster | ↑ | ↓ | Unchanged Aromatase | |||||||
| Kang et al., 2016 [100] | Human | ↓ | ↑ | – | ↓ | ↑CYP17, 3b-hsd2, cyp19 | ↑ Estrone | ||||
| PFNA | |||||||||||
| Halsne et al., 2016 [127] | Human | Normal acini maturation affected, ER-independent mechanisms to normal development of glandular breast tissue | |||||||||
| Benninghoff et al., 2011 [104] | Human | ↑ | |||||||||
| Maras et al., 2006 [128] | Human | ||||||||||
| Li et al., 2020 [129] | Human | ↑ | Altered expression of estrogen-responsive biomarker genes | ||||||||
| Ishibashi et al., 2008 [130] | Yeast | – | |||||||||
| Rosen et al., 2017 [112] | Human | ↑ | – | ||||||||
| Kjeldsen et al., 2013 [99] | Human and hamster | – | ↓ | Unchanged Aromatase | |||||||
| PFHxS | |||||||||||
| Li et al., 2020 [129] | Human | ↑ | Altered expression of estrogen-responsive biomarker genes | ||||||||
| Behr et al., 2018 [101] | Human | – | – | – | No effect on steroidogenesis | –: No effect on estrogen- or androgen-responsive genes | |||||
| Rosen et al., 2017 [112] | Human | ↑ | – | ||||||||
| Kjeldsen et al., 2013 [99] | Human and hamster | ↑ | ↓ | Unchanged Aromatase | |||||||
| PFDA | |||||||||||
| Halsne et al., 2016 [127] | Human | Normal acini maturation affected, ER-independent mechanisms to normal development of glandular breast tissue | |||||||||
| Benninghoff et al., 2011 [104] | Human | ↑ | |||||||||
| Li et al., 2020 [129] | Human | ↑ | Altered expression of estrogen-responsive biomarker genes | ||||||||
| Ishibashi et al., 2008 [130] | Yeast | – | |||||||||
| Kjeldsen et al., 2013 [99] | Human and hamster | – | ↓ | ↓ Aromatase | |||||||
| MIXTURE | |||||||||||
| Gogola et al., 2020 [126] | Human | ↓ | ↑↓ | ↑ 2-OHE1/E2 ratio | |||||||
| Human | ↑ | – | ↓2-OHE1, 16-OHE1, 2OHE1/E2 ratio, 16-OHE1/E2 ratio | ||||||||
| Gogola et al., 2020 [133] | Human | ||||||||||
| Human | Effect on IGF1 though ERα | ||||||||||
| Gogola et al., 2020 [133] | Human | – | – | –: Effects were independent of ER pathway | |||||||
| Human | – | – | –: Effects were independent of ER pathway | ||||||||
| Kjeldsen et al., 2013 [99] | Human and hamster | ↑ | ↓ | Unchanged Aromatase | |||||||
| Dairkee et al., 2018 [134] | Human | ↑ | ↓ | ||||||||
| Authors | Species | Body Weight | Organ Weight | Thyroid Hormone Level | Protein Expression/Level | Thyroid Cell Histology | Gene Expression | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T3 | T4 | TSH | TG | TSHR | TPO | ||||||
| PFOS | |||||||||||
| Chen et al., 2018 [135] | Zebrafish embryos | ↓ | ↓nuclear area | ↓ thyroid function-related gene expression | |||||||
| Du et al., 2013 [107] | Zebrafish embryos | ↑gene related to early thyroid development (hhex and pax8) | |||||||||
| Kim et al., 2011 [136] | Zebrafish embryos | ↓length | ↑ | ↓ | ↓TRα, TRβ, hhex, and pax8 | ||||||
| Ren et al., 2015 [137] | Amphibians (X. laevis) | ↑TH upregulated genes; ↓TH downregulated genes | |||||||||
| Shi et al., 2009 [138] | Zebrafish embryos | ↓ | ↑ | alter genes in HPT system (↓TSH, TTR, TRα, ↑TRβ) | |||||||
| Yu et al., 2011 [139] | Adult female Wistar rat | ↓ | ↓ | ↑hepatic genes related to T4 uptake and regulation | |||||||
| PFOS potassium salt (PFOS-K) | |||||||||||
| Chang et al., 2008 [140] | Female adult SD rat | ↓ | ↓TT4, transit ↑FT4 | ↓ | |||||||
| F-53B (PFOS substitute) | |||||||||||
| Deng et al., 2018 [141] | Zebrafish embryos | ↓ | ↑ | ↓ | ↑ttr, ↓tg | ||||||
| Hong et al., 2020 [142] | Adult female SD rat | ↓ | ↓ | ↑ | ↑ | Thyroid follicular hyperplasia | |||||
| PFOA | |||||||||||
| Blake et al., 2020 [43] | Pregnant CD-1 mice | ↓embryo | ↑placenta | ||||||||
| Godfrey et al., 2019 [143] | Japanese medaka embryo | ↑ thyroid-related genes | |||||||||
| Kim et al., 2021 [144] | Zebrafish embryos | ↑genes related to activation or metabolism | |||||||||
| HFPO-DA (PFOA substitute) | |||||||||||
| Blake et al., 2020 [43] | Pregnant CD-1 mice | ↑placenta | ↑placenta | ||||||||
| Conley et al., 2021 [145] | SD rat (dam) | ↓pup | ↓ | ↓ | |||||||
| PFNA | |||||||||||
| Liu et al., 2011 [146] | Zebrafish embryos | ↑ | alter genes related to TH synthesis and metabolism in F1 larvae | ||||||||
| PFHxS | |||||||||||
| Ramhøj et al., 2020 [147] | Wistar rat (dam and offspring) | ↓ | ↓ | – | |||||||
| Cassone et al., 2012 [148] | Chicken embryos | ↓embryo | ↓ | ↑TH-response genes | |||||||
| PFDA | |||||||||||
| Harris et al., 1989 [149] | Adult female C57BL/6 mice | ↓ | ↓Thymus | ↑ | ↑ | ||||||
| Authors | Cell Species | Protein | T-Screen | NIS | RAIU | Gene Expression | Molecular Docking | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TTR Binding | TBG | TPO | TR | T4 | |||||||
| PFOS | |||||||||||
| Ren et al., 2016 [150] | Human | B | |||||||||
| Song et al., 2012 [151] | Human | ↓ | |||||||||
| Selano et al., 2019 [152] | Rat male | ↑FT4, ↑hepatic uptake | |||||||||
| Xin et al., 2018 [153] | Human | B | – | B: TRα, TRβ | |||||||
| Human | ↑Transactivity | Fit into pocket of TTR and TRs | |||||||||
| Rat | ↑Compound alone | ||||||||||
| Weiss et al., 2009 [154] | Human | B | |||||||||
| Long et al., 2013 [155] | Rat | ↓Compound alone and +T3 | |||||||||
| Buckalew et al., 2020 [156] | Rat | ↓ | |||||||||
| Human | ↓ | ||||||||||
| Wang et al., 2019 [157] | Human | ↓ | |||||||||
| Song et al., 2011 [158] | Human | ↓ | |||||||||
| Du et al., 2013 [107] | Monkey | ↓Transactivity +T3 | |||||||||
| Human | Altered steroidogenic genes | ||||||||||
| Ren et al., 2015 [137] | Human | B: TRα | Fit into T3-binding pocket of TRα-LBD | ||||||||
| Rat | ↑Compound alone and +T3 | ||||||||||
| PFOS potassium salt PFOS-K | |||||||||||
| Buckalew et al., 2020 [156] | Rat | ↓ | |||||||||
| Human | ↓ | ||||||||||
| Wang et al., 2019 [157] | Human | ↓ | |||||||||
| F-53B PFOS substitute | |||||||||||
| Deng et al., 2018 [141] | Rat | ↑Compound alone | |||||||||
| PFOA | |||||||||||
| Ren et al., 2016 [150] | Human | B | – | ||||||||
| Song et al., 2012 [151] | Human | ↓ | |||||||||
| Selano et al., 2019 [152] | Rat male | ↑FT4, ↑hepatic uptake | |||||||||
| Weiss et al., 2009 [154] | Human | B | |||||||||
| Long et al., 2013 [155] | Rat | ↓Compound alone | |||||||||
| Buckalew et al., 2020 [156] | Rat | ↓ | |||||||||
| Human | ↓ | ||||||||||
| Ren et al., 2015 [137] | Human | B: TRα | Fit into T3-binding pocket of TRα-LBD | ||||||||
| Rat | – | ||||||||||
| Kim and Lee et al., 2021 [144] | Rat | Dio2↓ | |||||||||
| PFOA-ammonium | |||||||||||
| Buckalew et al., 2020 [156] | Rat | ||||||||||
| Human | ↓ | ||||||||||
| Wang et al., 2019 [157] | Human | ↓ | – | ||||||||
| PFNA | |||||||||||
| Ren et al., 2016 [150] | Human | B | – | ||||||||
| Weiss et al., 2009 [154] | Human | B | |||||||||
| Long et al., 2013 [155] | Rat | ↓Compound alone and +T3 | |||||||||
| Wang et al., 2019 [157] | Human | – | |||||||||
| Ren et al., 2015 [137] | Human | B: TRα | Fit into T3-binding pocket of TRα-LBD | ||||||||
| Rat | – | ||||||||||
| PFHxS | |||||||||||
| Ren et al., 2016 [150] | Human | B | – | ||||||||
| Weiss et al., 2009 [154] | Human | B | |||||||||
| Long et al., 2013 [155] | Rat | ↓Compound alone and +T3 | |||||||||
| Ren et al., 2015 [137] | Human | B: TRα weakly | Fit into T3-binding pocket of TRα-LBD | ||||||||
| Rat | – | ||||||||||
| PFHxS potassium PFHxS-K | |||||||||||
| Buckalew et al., 2020 [156] | Rat | ↓ | |||||||||
| Human | ↓ | ||||||||||
| PFDA | |||||||||||
| Long et al., 2013 [155] | Rat | ↓Compound alone | |||||||||
| Wang et al., 2019 [157] | Human | – | |||||||||
| Ren et al., 2015 [137] | Human | B: TRα | Fit into T3-binding pocket of TRα-LBD | ||||||||
| Rat | – | – | |||||||||
| Ren et al., 2016 [150] | Human | B | – | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gundacker, C.; Audouze, K.; Widhalm, R.; Granitzer, S.; Forsthuber, M.; Jornod, F.; Wielsøe, M.; Long, M.; Halldórsson, T.I.; Uhl, M.; et al. Reduced Birth Weight and Exposure to Per- and Polyfluoroalkyl Substances: A Review of Possible Underlying Mechanisms Using the AOP-HelpFinder. Toxics 2022, 10, 684. https://doi.org/10.3390/toxics10110684
Gundacker C, Audouze K, Widhalm R, Granitzer S, Forsthuber M, Jornod F, Wielsøe M, Long M, Halldórsson TI, Uhl M, et al. Reduced Birth Weight and Exposure to Per- and Polyfluoroalkyl Substances: A Review of Possible Underlying Mechanisms Using the AOP-HelpFinder. Toxics. 2022; 10(11):684. https://doi.org/10.3390/toxics10110684
Chicago/Turabian StyleGundacker, Claudia, Karine Audouze, Raimund Widhalm, Sebastian Granitzer, Martin Forsthuber, Florence Jornod, Maria Wielsøe, Manhai Long, Thórhallur Ingi Halldórsson, Maria Uhl, and et al. 2022. "Reduced Birth Weight and Exposure to Per- and Polyfluoroalkyl Substances: A Review of Possible Underlying Mechanisms Using the AOP-HelpFinder" Toxics 10, no. 11: 684. https://doi.org/10.3390/toxics10110684





